Abstract
Purpose
To report the long-term follow-up results after cyclocryotherapy, applied to the 3-o'clock and 9-o'clock positions in blind refractory glaucoma patients.
Methods
We retrospectively reviewed the charts of 19 blind patients, and a total of 20 eyes with refractory glaucoma who were treated with cyclocryotherapy. Cyclocryotherapy treatments were performed using a retinal cryoprobe. The temperature of each cyclocryotherapy spot was -80℃ and each spot was maintained in place for 60 seconds. Six cyclocryotherapy spots were placed in each quadrant, including the 3-o'clock and 9-o'clock positions.
Results
The mean baseline pretreatment intraocular pressure (IOP) in all eyes was 50.9 ± 12.5 mmHg, which significantly decreased to a mean IOP at last follow-up of 14.1 ± 7.1 mmHg (p < 0.001). The mean number of antiglaucoma medications that patients were still taking at last follow-up was 0.3 ± 0.6. Devastating post-procedure phthisis occurred in only one eye.
Cyclodestruction therapy decreases intraocular pressure (IOP) by destroying ciliary bodies, thereby reducing aqueous humor production. Cyclocryotherapy was initially introduced by Bietti [1] in 1950 to reduce IOP. However, it was not a popular therapy until the late 1960s, when de Roetth [2] reported a success rate of 73% with cyclocryotherapy in 165 adult patients with uncontrolled IOP. Currently, this procedure is mainly reserved for end-stage or very severe cases of refractory glaucoma. Also, because of the variable success rates reported in adults (34% to 92%), and the significant postoperative pain and complications associated with cyclocryotherapy including phthisis and retinal detachment, transscleral neodymium: YAG (Nd: YAG) and diode lasers are replacing cyclocryotherapy as the preferred form of cyclodestruction in advanced patients [3,4,5]. When undergoing transscleral treatments to the ciliary region, the long posterior ciliary arteries and nerves lying in the suprachoroidal space and the conducting sensory impulses from the cornea are susceptible to damage. Therefore, treatment to the 3-o'clock and 9-o'clock positions has always been avoided to prevent damage to the long posterior ciliary arteries and nerves, which can lead to development of phthisis. However, Streiff and Stucchi [6] described a procedure to destroy long posterior ciliary vessels in order to minimize increases in IOP, a method that they termed cycloanemization. They reported an overall success rate of 57% to 64% in different types of glaucoma.
The purpose of this study was to report the long-term follow-up results after cyclocryotherapy was performed on the quadrants and the 3-o'clock and 9-o'clock positions of blind refractory glaucoma patients'eyes.
We retrospectively reviewed the medical records of all refractory glaucoma patients treated with cyclocryotherapy from 2006 to 2008 at our clinic. Among these patients, we selected 27 who underwent cyclocryotherapy in each quadrant and the 3-o'clock and 9-o'clock positions between the rectus muscles. The inclusion criteria included presence of primary, secondary, or neovascular glaucoma associated with high IOP that was unresponsive to other medical or surgical treatment for at least three months. Among these, eight patients were excluded because their follow-up period was less than three months. Finally, twenty eyes were included in this study. Because cyclocryotherapy can have severe complications (visual loss and phthisis), we selected only refractory glaucoma patients that had no vision. The aim of treatment in all patients was reduction of IOP and control of ocular pain or headaches. All patients had given informed consent for cyclocryotherapy.
All procedures were performed by the same surgeon (SJK). Cryotherapy treatments were performed using a retinal cryoprobe (2.5-mm-diameter) while the patient was under retrobulbar anesthesia. The cryoprobe was placed 1.5 to 3 mm posterior to the limbus. The temperature of each cyclocryotherapy spot was -80℃ and was maintained in place for 60 seconds. Six cyclocryotherapy applications were performed at each quadrant between the rectus muscles and at the 3-o'clock and 9-o'clock positions. A film of ice that was 1.5 times of the size of the surface area of the cryoprobe formed at each application site and then thawed for one minute. The interval between the end of the thaw and the beginning the next procedure was about 60 seconds. All patients received a subconjunctival steroid injection at the conclusion of the procedure. Topical steroid and cycloplegics were tapered during the first few weeks after treatment. Most patients continued pretreatment glaucoma medications, and then use of these medication was were tapered after treatment, as allowed.
Antiglaucoma medications use and IOP were recorded at each pre-procedure and post-procedure patient visit. IOP was measured using the Goldmann tonometer. Any observations of excessive anterior segment inf lammation (corneal infiltrations, hypopyon, marked conjunctival edema, or injection) were recorded. Any reports of significant postprocedure pain were also noted. Criteria for successful outcome from cyclocryotherapy included IOP lower than 21 mmHg with or without antiglaucoma medications and relief from ocular pain or headache. The devastating complications that occurred in this study included retinal detachment and development of phthisis bulbi.
All data are presented as a mean ± standard deviation unless otherwise specified. For statistical analyses, the Student's t-test and the chi-square test was used for paired and unpaired data. SPSS ver. 14.0 (SPSS Inc., Chicago, IL, USA) was used for all statistical analyses, and p-values <0.05 were considered statistically significant.
Nineteen patients (20 eyes) with inadequately controlled, refractory glaucoma were treated with cyclocryotherapy performed at each quadrant and the 3-o'clock and 9-o'clock positions between the rectus muscles. The mean age of the 19 subjects was 67.3 ± 14.9 years (range, 27 to 85 years); 10 were women and nine were men. The average follow-up time was 27.1 months (range, 24 to 34 months). Symptoms leading patients to seek cyclocryotherapy treatment included ocular pain in 14 eyes and headache in six eyes (Table 1).
The most common pre-procedure diagnosis was neovascular glaucoma in 14 eyes, followed by primary open angle glaucoma in five eyes, and chronic angle closure glaucoma in one eye. Causes of neovascular glaucoma were diabetic retinopathy in four eyes, central retinal vein occlusion in four eyes, ocular ischemic syndrome in four eyes, central retinal artery occlusion in one eye and retinal detachment in one eye (Table 1). Prior ocular treatment had been performed in seven eyes: panretinal photocoagulation in four eyes, trabeculectomy in two eyes, and vitrectomy was performed in one eye because of retinal detachment. Cyclocryotherapy was performed 5.5, 13.3, and 6 years after each type of prior treatment, respectively. For the remaining 13 eyes, no procedure was performed prior to cyclocryotherapy.
The mean IOP in all patients was 50.9 ± 12.5 mmHg pretreatment and 18.4 ± 6.6 mmHg at the last follow-up. The mean IOP at last follow-up was significantly reduced, by 32.4 mmHg (73.9%, p < 0.001). In the neovascular glaucoma patient group, the mean IOP decreased by 33.4 mmHg (63.1%, p < 0.001). In the primary open angle glaucoma patient group, the mean IOP decreased by 26.4 mmHg (69.1%, p < 0.001). In one case of chronic angle closure glaucoma, the IOP decreased by 54 mmHg (90%). The mean number of preoperative antiglaucoma medications being taken prior to therapy was 2.4 ± 1.0, and this number significantly decreased to 0.4 ± 0.5 at final follow-up (p < 0.001). In the neovascular glaucoma patient group, a mean of 2.4 ± 1.0 antiglaucoma medications were taken, and this number reduced to 0.4 ± 0.7 by the last follow-up (p < 0.001). The primary open angle glaucoma patient group used a mean of 2.8 ± 0.5 medications prior to treatment, and this number reduced to 0.4 ± 0.9 (p < 0.001). The one chronic angle closure glaucoma patient used two medications prior to surgery, but no medication was used after the procedure. The neovascular glaucoma group had an 84.6% success rate, and the primary open angle glaucoma and the chronic angle closure glaucoma groups each showed 100% success rates. Overall, the success rate in all patients following cyclocryotherapy was 90% (Table 2).
Postoperative complications developed in five eyes including two cases of hyphema, two cases of uveitis, and one case of phthisis. Hyphema and severe uveitis gradually resolved using topical antibiotics and steroids. A devastating complication (phthisis) occurred in only one eye.
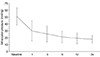
The change in mean IOP over the follow-up period is shown in Fig. 1. Mean IOP was 25.3 ± 11.8 mmHg at one month post-procedure, 25.3 ± 10.5 mmHg at three months post-procedure, 21.1 ± 8.2 mmHg at six months post-procedure, and 17.8 ± 5.7 mmHg at 24 months post-procedure (Fig. 1). The mean number of IOP-control agents was 2.4 ± 1.0 before treatment, 0.56 ± 0.8 at one month post-procedure, 0.56 ± 0.69 at three months post-procedure, and 0.43 ± 0.69 at 24 months post-procedure (Fig. 2). In Fig. 3, Kaplan-Meier survival curves show the cumulative success rates of cyclocryotherapy treatment included applications to the 3-o'clock and 9-o'clock positions. The cumulative success rates of cyclocryotherapy were 98.6% at one month, 95.3% at three month, 92.6% at six month, 91.0% at one year, 90.0% at two years, post-procedure.
In this study, we reported the clinical outcomes for 19 patients (20 eyes) treated with cyclocryotherapy performed at each quadrant and the 3-o'clock and 9-o'clock positions between the rectus muscles. The mean IOP at last follow-up was significantly reduced by 32.4 mmHg (73.9%, p < 0.001) from the baseline IOP, and the mean number of antiglaucoma medications taken by the study patients decreased to 0.4 ± 0.5 (p < 0.001) at final follow-up. The overall success rate was approximately 90%. After treatment, acute complications developed in five eyes, including two cases of hyphema and two cases of uveitis, but these complications gradually resolved with medication. Devastating phthisis occurred only in one eye.
Ciliary body destruction is a treatment reserved for eyes that do not respond to other forms of surgical or medical treatment. Ciliary body ablation has been performed with many techniques, including ciliary body excision, penetrating cyclodiathermy, cyclocryotherapy, microwave cyclodestruction, and trans-scleral and endoscopic cyclophotocoagulation. There are considerable differences in efficacy and complication rates between these treatment modalities [1,7,8,9,10,11]. Among these methods, cyclocryotherapy is used to treat uncontrolled IOP in eyes of patients with refractory glaucoma. However, success rates vary widely from 34% reported by Krupin et al. [5] to 92% reported by Bellows and Grant [12]. After performing cyclocryotherapy on 68 eyes in 64 patients, Benson and Nelson [13] reported a 29.4% success rate. Hennekes and Belgrado [14] reported a 58.3% success rate from 56 cases, while Goldenberg-Cohen et al. [15] achieved a 60.5% success rate from 38 cases.
Complications that may develop after performing cyclocryotherapy include temporary IOP increase, uveitis, pain, hyphema, choroidal detachment, vitreous hemorrhage, anterior segment ischemia, subretinal fibrosis, subluxated lens, vision loss, low IOP, and phthisis bulbi [13]. Some of the most serious complications include vision loss, low IOP, and phthisis bulbi. Benson and Nelson [13] reported phthisis bulbi in 11.8% of their cases (follow-up range of 2.6 to 6.3 years), while Caprioli et al. [16] reported 12% and 6% of their patients developed phthisis bulbi after cyclocryotherapy was performed in 360 degrees and 180 degrees, respectively (follow-up period of 29 months for both therapy protocol). In 1998, Heuring et al. [17] reported a 6.7% rate of phthisis bulbi development in 76 eyes from 75 patients (follow-up range of 12 to 36 months). The success rate of this study (90%) was higher than that of previous studies, and the retreatment rate (0%) was very low. Also, a severe complication (phthisis bulbi) developed in only one case. Our study's means follow-up time was 27.1 months. Our cyclocryotherapy protocol, which included the 3-o'clock and 9-o'clock positions for destroying long posterior ciliary vessels, was more effective for reducing IOP in case of neovascular glaucoma than other protocols. In particular, our cyclocloanemization technique to destroy long posterior ciliary vessels lead to slower progress toward neovascularization than in previous studies. Perhaps because our study included only six cyclocryotherapy applications, we achieved fewer severe complications than other studies. We avoided other complications (vision loss) because we only selected blind patients. Given these results, we conclude that six cyclocryotherapy application, including the 3-o'clock and 9-o'clock positions, are effective to control IOP in blind refractory glaucoma patients.
Cyclocryotherapy decreases aqueous production by damaging the ciliary epithelium with a freezing technique and cut off the vascular supply to the ciliary body [18]. Upon completion of cyclocryotherapy, the blood supply in the ciliary epithelium and ciliary body is inhibited, resulting in reduction of aqueous fluid production. The effectiveness of cyclocryotherapy is dependent on the tissue temperature at the moment of performing the procedure. Intracellular ice crystals begin to form at temperatures below -15℃. To induce permanent cell damage by intracellular changes, a freezing time of 30 seconds or longer is necessary. For most cases, a 60-second freezing time at -80℃ has been found to beoptimal [16,19,20]. According to previous studies, performing cryotherapy on 180° or more of the eye at once is not recommended, and application to the 3-o'clock and 9-o'clock positions, where the long posterior ciliary artery is located, should be avoided [12,13]. These recommendations result from the association of cyclocryotherapy with the development of complications, such as phthisis bulbi. However, Streiff and Stucchi [6] described a procedure, termed cycloanemization, where they of destroyed long posterior ciliary vessels, and they reported overall success rates in the range of 57% to 64% for different types of glaucoma. According to that study, a mean IOP reduction of 38.5 mmHg was achieved by the time of the last follow- up, and 19 out of the total 20 had an IOP of showed 21 mmHg or lower at their last follow-up. Our study's the higher overall success rate compared to previous studies may be explained by this cycloanemic effect.
In our study, most of the cases we examined (84.5%) were caused by neovascular glaucoma, while this type of glaucoma accounted for only 51% of cases in the study by Brindley and Shields [21], and only 21% of the cases reported by Caprioli et al. [16]. Because of the natural history of neovascular glaucoma and its poor prognosis, success rates following treatment are lower and the recurrence and retreatment rates are higher than for other types of glaucoma [13,17]. Our findings indicate that cyclocryotherapy that includes application at the 3-o'clock and 9-o'clock positions is effective and has better long-term clinical outcomes for neovascular glaucoma patients.
Corneal sensitivity is reduced after intense photocoagulation of the peripheral retina, combined retinal cryocoagulation, an encirclement procedure for treatment of retinal detachment, and cryocoagulation of the ciliary body [22,23]. During transscleral treatments of the ciliary region, the long posterior ciliary nerves lying in the suprachoroidal space and conducting sensory impulses from the cornea are susceptible to damage [24]. Benson and Nelson [13] reported that cyclocryotherapy rendered 71.4% of painful eyes comfortable despite poor prior IOP control. They suggested that pain relief from cyclocryotherapy was not due solely to pressure control. In this study, relief from ocular pain and headaches was achieved for all 20 cases. Therefore, we conclude that cyclocryotherapy that includes applications to the 3-o'clock and 9-o'clock positions may have positive effects on long posterior ciliary nerves and may help relieve pain.
In summary, we believe that cyclocryotherapy performed at each quadrant and at the 3-o'clock and 9-o'clock positions between the rectus muscles results in good pressure control and pain relief for various types of glaucoma, especially neovascular glaucoma. Our study has several limitations. First, retrospective data collection may decrease the reliability of the data. Also, we only selected refractive glaucoma patients who have no vision, leading to a possibility of selection bias. Second, this study includes a relatively small number of subjects, which decreases our statistical power. Third, our study lacks a control group. We recommend confirmation of the reliability of our results with well-designed prospective studies that have a control group.
Figures and Tables
Fig. 2
Mean number of glaucoma medications per patient over the follow-up period. The mean number of intraocular pressure-control agents was 2.4 ± 1.0 before surgery, 0.56 ± 0.8 at one-month post-procedure, 0.56 ± 0.69 at three-months post-procedure, and 0.43 ± 0.69 at the final follow-up (24 months post-procedure).

Fig. 3
Kaplan-Meier survival curves showing the cumulative success rates of cyclocryotherapy applied to the 3-o'clock and 9-o'clock positions. Success was defined as IOP lower than 21 mmHg with or without antiglaucoma medications. The cumulative success rates of cyclocryotherapy were 98.6% at one month, 95.3% at three months, 92.6% at six months, 91.0% at one year, 90.0% at two years post-procedure.

References
1. Bietti G. Surgical intervention on the ciliary body: new trends for the relief of glaucoma. J Am Med Assoc. 1950; 142:889–897.
2. De Roetth A Jr. Cryosurgery for the treatment of advanced chronic simple glaucoma. Am J Ophthalmol. 1968; 66:1034–1041.
3. Brancato R, Carassa RG, Bettin P, et al. Contact transscleral cyclophotocoagulation with diode laser in refractory glaucoma. Eur J Ophthalmol. 1995; 5:32–39.
4. Hampton C, Shields MB, Miller KN, Blasini M. Evaluation of a protocol for transscleral neodymium: YAG cyclophotocoagulation in one hundred patients. Ophthalmology. 1990; 97:910–917.
5. Krupin T, Mitchell KB, Becker B. Cyclocryotherapy in neovascular glaucoma. Am J Ophthalmol. 1978; 86:24–26.
6. Streiff EB, Stucchi C. Antiglaucomatous cycloanemization: review of 207 operations performed during eight years. Am J Ophthalmol. 1966; 61(5 Pt 2):1325–1329.
7. Sautter H, Demeler U. Antiglaucomatous ciliary body excision. Am J Ophthalmol. 1984; 98:344–348.
8. Vogt A. Cyclodiathermypuncture in cases of glaucoma. Br J Ophthalmol. 1940; 24:288–297.
9. Walton DS, Grant WM. Penetrating cyclodiathermy for filtration. Arch Ophthalmol. 1970; 83:47–48.
10. Zarbin MA, Michels RG, de Bustros S, et al. Endolaser treatment of the ciliary body for severe glaucoma. Ophthalmology. 1988; 95:1639–1648.
11. Finger PT, Perry HD, Shakin JL, et al. Microwave cyclodestruction: evaluation on human eyes. Br J Ophthalmol. 1995; 79:678–682.
12. Bellows AR, Grant WM. Cyclocryotherapy of chronic open-angle glaucoma in aphakic eyes. Am J Ophthalmol. 1978; 85(5 Pt 1):615–621.
13. Benson MT, Nelson ME. Cyclocryotherapy: a review of cases over a 10-year period. Br J Ophthalmol. 1990; 74:103–105.
14. Hennekes R, Belgrado G. Cyclocryotherapy as an alternative treatment for primary glaucoma. Bull Soc Belge Ophtalmol. 1992; 244:169–176.
15. Goldenberg-Cohen N, Bahar I, Ostashinski M, et al. Cyclocryotherapy versus transscleral diode laser cyclophotocoagulation for uncontrolled intraocular pressure. Ophthalmic Surg Lasers Imaging. 2005; 36:272–279.
16. Caprioli J, Strang SL, Spaeth GL, Poryzees EH. Cyclocryotherapy in the treatment of advanced glaucoma. Ophthalmology. 1985; 92:947–954.
17. Heuring AH, Hutz WW, Hoffmann PC, Eckhardt HB. Cyclocryotherapy in neovascular glaucoma and non-neovascular glaucoma. Klin Monbl Augenheilkd. 1998; 213:213–219.
18. Ferry AP. Histopathologic observations on human eyes following cyclocryotherapy for glaucoma. Trans Sect Ophthalmol Am Acad Ophthalmol Otolaryngol. 1977; 83:90–113.
19. Bellows AR. Cyclocryotherapy for glaucoma. Int Ophthalmol Clin. 1981; 21:99–111.
20. Prost M. Cyclocryotherapy for glaucoma: evaluation of techniques. Surv Ophthalmol. 1983; 28:93–100.
21. Brindley G, Shields MB. Value and limitations of cyclocryotherapy. Graefes Arch Clin Exp Ophthalmol. 1986; 224:545–548.
22. Gibson RA. Reduction of corneal sensitivity after retinal detachment surgery. Br J Ophthalmol. 1981; 65:614–617.
23. Martin XY, Safran AB. Corneal hypoesthesia. Surv Ophthalmol. 1988; 33:28–40.
24. Muller LJ, Vrensen GF, Pels L, et al. Architecture of human corneal nerves. Invest Ophthalmol Vis Sci. 1997; 38:985–994.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download