Abstract
Purpose
To compare clinical, angiographic, and optical coherence tomographic characteristics between eyes with acute Vogt-Koyanagi-Harada (VKH) disease and eyes with acute bilateral central serous chorioretinopathy (CSC), and to demonstrate distinguishing features between the two diseases in confusing cases.
Methods
The medical records of 35 patients with VKH disease and 25 patients with bilateral CSC were retrospectively reviewed. Characteristics according to slit-lamp biomicroscopy, ophthalmoscopy, fundus photography, fluorescein angiography, indocyanine green angiography, and spectral-domain optical coherence tomography were compared between the two diseases.
Results
Five of 35 patients (10 of 70 eyes, 14.3%) with VKH disease were initially misdiagnosed as CSC patients, and six of 25 patients (12 of 50 eyes, 24%) with bilateral CSC were initially misdiagnosed as patients with VKH disease. Pigment epithelial detachment in CSC and optic disc hyperemia in VKH disease show the highest positive predictive values of 100% for each disease.
Vogt-Koyanagi-Harada (VKH) disease is characterized by bilateral granulomatous uveitis, and is often associated with exudative retinal detachment, and with extraocular manifestations such as meningismus, tinnitus, alopecia, and vitiligo [1]. Central serous chorioretinopathy (CSC) is characterized by serous detachment of the neurosensory retina associated with choroidal vascular hyperpermeability [23]. VKH disease and CSC are considered to be completely different diseases with different pathophysiologies.
The acute stages of both diseases are sometimes confused with one another, particularly when the diseases manifest bilateral lesions such as bilateral subretinal fluid, multifocal leakages on fluorescein angiography (FA), and bilateral thick choroid on enhanced depth imaging optical coherence tomography (OCT) [45678]. Yang et al. [9] recently reported that 90 of 410 patients (22.0%) with VKH disease had been initially misdiagnosed as patients with CSC in other hospitals. Also, CSC may clinically mimic VKH disease, particularly in atypical cases with unusually large, bilateral bullous exudative retinal detachment [1011].
In VKH disease, early and aggressive treatment with corticosteroids, followed by slow tapering of treatment over three to six months, has shown favorable anatomical and visual results. Delayed diagnosis, which is defined as diagnosis more than one month after a patient's first symptoms and signs of VKH, could cause the disease in its acute phase to evolve into chronic recurrent disease [12]. In CSC, on the other hand, in cases of high levels of endogenous and exogenous corticosteroids, correction to normal levels of corticosteroids could lead to resolution of detachment in 90% of cases [13]. Without adequate management of CSC, atrophy of photoreceptors in the fovea-a contributing factor to irreversible visual loss-could occur as early as four months after the onset of symptoms [1415].
The present study compares clinical, angiographic, and optical coherence tomographic characteristics between eyes with acute VKH disease and eyes with acute bilateral CSC. By reviewing confusing cases, we demonstrate specific features that are useful in distinguishing between the two diseases in instances of bilateral lesions.
This retrospective observational study was approved by the institutional review board of Yonsei University, Seoul, Korea. We included treatment-naive patients with acute VKH disease or acute bilateral CSC from June 2006 to October 2014. At presentation, all 60 patients underwent a comprehensive ophthalmic examination, including best-corrected visual acuity (BCVA), slit-lamp biomicroscopy, ophthalmoscopy, fundus photography, and FA (HRA2; Heidelberg Engineering, Dossenheim, Germany). Indocyanine green angiography (ICGA) was performed on 32 eyes (45.7%) with VKH disease, and on 28 eyes (56%) with bilateral CSC. Spectralis OCT ver. 1.5.12.0 (Heidelberg Engineering) was performed on 50 eyes (71.4%) with VKH disease, and on all eyes with bilateral CSC.
Diagnosis of VKH disease was based on the revised diagnostic criteria for VKH by an international committee for nomenclature in ophthalmology (2001) [16]. Diagnosis of CSC was made in instances of serous detachment of the neurosensory retina with typical fluorescein leakage patterns [17]. Inflammatory cell reaction was determined by cells in the anterior chamber or the vitreous cavity with slit-lamp biomicroscopy. Optic disc hyperemia was determined by fundus photography. Choroidal vascular hyperpermeability was defined as multifocal hyperfluorescences in the middle and late phases of ICGA [18]. Signs of hypofluorescent dots were defined as multiple dark spots in late-phase ICGA [1920]. Measures of BCVA were converted to the logarithm of the minimum angle of resolution (log-MAR) equivalents.
Subretinal fluid was defined as homogenous hyporeflective space between the neurosensory retina and retinal pigment epithelium (RPE) of a patient on OCT. Pigment epithelium detachment (PED) was defined as a dome-shaped elevation of RPE with hyporeflectivity, bound inferiorly by a Bruch's membrane. Subretinal septa was defined as a highly reflective line, separated from the inner and outer segments of photoreceptors in attached areas of the retina [21]. RPE folds were defined by the presence of at least two sets of peaks and troughs of RPE [22]. OCT findings were evaluated by two independent readers (WBS and MKK), in blinded clinical diagnosis.
The central macular thickness (CMT) of patient eyes was measured by OCT, and was defined as a mean retinal thickness of 1 mm at the center, as described in the Early Treatment Diabetic Retinopathy Study [23]. Choroidal thickness was measured by enhanced depth imaging OCT, which was performed by positioning the objective lens of the Spectralis OCT scanner close enough to invert the image, as previously described [24]. Subfoveal choroidal thickness was defined as the vertical distance from the hyperreflective line of Bruch's membrane at the fovea to the outermost hyperreflective line of the chorioscleral interface, and was measured using the calipers provided by software from the Heidelberg Spectralis OCT.
A chi-square test was used for categorical analysis, and two-sample t-tests were used for analysis of continuous variables. Fisher's exact tests and Mann-Whitney U-tests were used if the expected cell count was lower than five. Data were expressed as mean ± standard deviation. A p-value less than 0.05 was considered significant. All statistical analyses were performed with SPSS ver. 18.0 (SPSS Inc., Chicago, IL, USA).
Seventy eyes from 35 patients with acute VKH disease, and 50 eyes from 25 patients with acute bilateral CSC were included in this study. The mean patient age was 43.5 ± 10.7 years (range, 21 to 62 years) in the VKH group, and 51.1 ± 12.1 years (range, 32 to 84 years) in the CSC group (p = 0.027). The study population showed male predominance (68%) in the CSC group, whereas the VKH group was predominately female (62.9%) (p = 0.035). Among all 60 patients, headaches were a more frequent symptom in the VKH group (54.3%) than in the CSC group (8%) (p < 0.001). Tinnitus occurred more frequently among patients in the VKH group (25.7%), although the comparison was not significant (Table 1).
In a mean follow-up period of 26 ± 21 months among VKH patients, final measures of BCVA showed significant improvement from 0.81 ± 0.62 to 0.15 ± 0.20 logMAR (p < 0.001). In bilateral CSC patients, however, final BCVA values did not show significant improvement (p = 0.066). Initial BCVA values were significantly worse in the VKH group (0.81 ± 0.62 logMAR) than in the bilateral CSC group (0.29 ± 0.34 logMAR) (p < 0.001), but there were no significant differences in the final BCVA values of either group. Inflammatory cells in the anterior chamber or vitreous cavity were more frequently observed among patients in the VKH group (58 of 70 eyes, 82.9%) than in the CSC group (2 of 50 eyes, 4.0%) (p < 0.001). Optic disc hyperemia was observed in 54 of 70 eyes (77.1%) in the VKH group, whereas none of the eyes of CSC patients showed disc hyperemia. During late-phase FA, multifocal leakages were observed in 65.7% of patient eyes in the VKH group and 90% of patient eyes in the CSC group (p = 0.001), and disc leakage was more frequently observed in the VKH group (85.7%) than in the CSC group (2.0%). During late-phase ICGA, choroidal vascular hyperpermeability was more frequently observed in the CSC group (89.3%), whereas hypofluorescent dots were more frequently observed in the VKH group (93.8%) (Table 2, Fig. 1A-1F, Fig. 2A-2F).
In comparing OCT findings between the VKH and CSC groups, PED was more frequently observed in the CSC group (p < 0.001), but subretinal septa and RPE folds were more frequently observed in the VKH group ( p < 0.001 and p < 0.001, respectively) (Fig. 1G and 1H, Fig. 2G and 2H). Mean CMT was 590 ± 309 µm in the VKH group and 405 ± 158 µm in the CSC group, and the VKH group had significantly thicker measures of CMT (p = 0.005). Initial subfoveal choroidal thickness was not measurable in 24 of the 50 eyes of patients (48%) with VKH disease, because the choroidal layer at presentation was thicker than the depth range of the Spectralis OCT (approximately 1,000 µm) [7]. Even after exception of the non-measurable cases in the eyes of VKH patients, the mean subfoveal choroidal thickness was 628 ± 309 µm in the VKH group and 465 ± 105 µm in the CSC group. Patients in the VKH group had significantly thicker measures of subfoveal choroids (p < 0.001).
Five of 35 patients (10 of 70 eyes, 14.3%) with VKH disease were initially misdiagnosed as having CSC, because six eyes (60%) did not show inflammatory cell reactions, and eight eyes (80%) did not show subretinal septa or RPE folds on OCT. Additionally, six of 25 patients (12 of 50 eyes, 24%) with bilateral CSC were initially misdiagnosed as having VKH disease, because three patients (50%) had headaches or tinnitus. Optic disc hyperemia was observed in 54 of 120 eyes, and all 54 of these eyes were finally diagnosed with VKH, showing a 100% positive predictive value of optic disc hyperemia for VKH. What is more, PED was observed in 30 of 120 eyes, and all 30 of these eyes were finally diagnosed with CSC, demonstrating the 100% positive predictive value of PED in CSC. In analyzing each clinical manifestation, optic disc hyperemia and disc leakage on FA showed the highest positive predictive values in VKH, and PED showed the highest positive predictive value in bilateral CSC.
Thirty-four of 35 patients (97%) with VKH were treated with systemic corticosteroid of more than 0.75 mg/kg, followed by gradual tapering over three to six months. Subsequently, three patients progressed into a chronic recurrent stage of the disease. Among the patients with bilateral CSC, focal laser photocoagulation was performed as an initial treatment in 13 of 50 eyes (26%), photodynamic therapy was performed in six eyes (12%), anti-vascular endothelial growth factor was administered in 22 eyes (44%), and five eyes (10%) were observed without treatment. Two patients with bilateral CSC received systemic steroid treatment as an initial treatment because of incorrect diagnosis.
This study compares clinical, angiographic, and optical coherence tomographic characteristics between eyes with acute VKH disease and eyes with acute bilateral CSC. Subsequently, we review initially-misdiagnosed VKH or CSC cases in order to identify specific features that facilitate distinction between the two diseases. Patients with VKH disease were misdiagnosed at presentation as having CSC in 14.3% of patients in this study, which is similar to the rate of misdiagnosis of 16.7% in previous reports [9]. Incorrect diagnoses sometimes occur when patients do not show inflammatory cell reactions on slit-lamp ophthalmoscopy, and do not have typical subretinal septa or RPE folds on OCT. However, PED is a good distinguishing point, because PED is not observed in any cases of VKH disease herein. On the other hand, patients with bilateral CSC were initially misdiagnosed as having VKH disease in 24% of our patients, particularly when the patients manifested headaches or tinnitus. In this case, optic disc hyperemia and disc leakage on FA are good distinguishing points, because these manifestations are not shown in the eyes of any CSC patients in this study.
Optic disc hyperemia a nd disc leakage on FA a re frequently observed in the eyes of patients VKH disease, but are not observed in the group of CSC patients in this study. Indeed, these signs are related to the pathophysiology of VKH disease as an inflammatory disease. Because the optic disc is known to be an intraocular structure that is sensitive to posterior segment inflammation, the acute stage of VKH disease shows optic disc changes, such as disc staining or disc leakage on FA [25]. On the other hand, PED is frequently observed in the group of patients with CSC, and is not observed in the patients with VKH disease in this study. This is related to the pathophysiology of CSC. Increased hydrostatic pressure in the choroids of CSC eyes causes detachment of the pigment epithelium (i.e., PED), and the subsequent breakdown of RPE tight junctions induces subretinal fluid from this choroidal fluid [26]. Therefore, in confusing cases, the observation of optic disc hyperemia or disc leakages on FA in VKH disease, and PED on OCT in CSC could be helpful in making an accurate distinction between the two diseases.
Although inf lammatory cell reactions and subretinal septa or RPE folds on OCT were frequently observed in patients with VKH disease herein, these manifestations are not always observed in patients VKH disease. Therefore, we cannot rule out VKH disease when patients do not manifest inflammatory cells. In addition, because headaches and tinnitus are nonspecific symptoms, these symptoms alone cannot confirm VKH disease.
Without prompt and adequate corticosteroid treatment for patients with VKH, inflammation of the posterior segment may progress into anterior uveitis, and in turn, the anterior uveitis may proceed to recurrent or chronic granulomatous uveitis in VKH patients [927]. In CSC eyes, however, the use of corticosteroid potentially leads to exacerbation of the disease, and may result in permanent visual loss due to subretinal fibrosis and macular scar formation [11282930]. Accordingly, correct diagnosis of each disease at the initial presentation of patients is essential to improve the visual outcomes of patients with VKH or CSC.
This study compares clinical, angiographic, and optical coherence tomographic characteristics between eyes with acute VKH disease and eyes with acute bilateral CSC. In conclusion, optic disc hyperemia in VKH disease and PED in bilateral CSC are the most specific clinical manifestations of each disease at initial patient presentation.
Figures and Tables
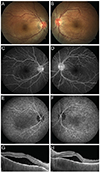 | Fig. 1Representative case of acute Vogt-Koyanagi-Harada disease. (A,B) On fundus examination, bilateral serous submacular fluids and hyperemic discs are observed. (C,D) Late-phase fluorescence angiography shows multiple hyperfluorescent spots around the discs, and disc hyperfluorescence in both eyes. (E,F) Indocyanine green angiography shows hyperfluorescent dark dots throughout posterior poles in both eyes. (G,H) Optical coherence tomography reveals subretinal fluid with thickened choroids. |
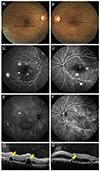 | Fig. 2Representative case of acute bilateral central serous chorioretinopathy. (A,B) On fundus examination, bilateral serous submacular fluids are observed. (C,D) Late-phase fluorescence angiography shows multifocal hyperfluorescent dots throughout posterior poles in both eyes. (E,F) Late-phase indocyanine green angiography shows multifocal hyperf luorescence, suggesting choroidal vascular hyperpermeability. (G,H) Optical coherence tomography (OCT) reveals subretinal fluid and thickened choroids. Additionally, OCT scans reveal pigment epithelial detachments (yellow arrows), corresponding to black lines in (A) and (B). |
Table 1
Baseline characteristics of patients with acute Vogt-Koyanagi-Harada disease and acute bilateral central serous chorioretinopathy

Table 2
Comparison of clinical, angiographic, and optical coherence tomographic features between eyes with acute Vogt-Koyanagi-Harada disease and eyes with acute bilateral central serous chorioretinopathy

Values are presented as mean ± SD or number (%); ICGA was performed in 32 eyes with VKH disease, and 28 eyes with CSC; Optical coherence tomography was performed in 50 eyes with VKH disease, and all of the CSC eyes.
VKH = Vogt-Koyanagi-Harada disease; CSC = central serous chorioretinopathy; BCVA = best-corrected visual acuity; logMAR = logarithm of the minimum angle of resolution; FA = fluorescein angiography; ICGA = indocyanine green angiography; RPE = retinal pigment epithelium.
References
1. Moorthy RS, Inomata H, Rao NA. Vogt-Koyanagi-Harada syndrome. Surv Ophthalmol. 1995; 39:265–292.
2. Spaide RF, Goldbaum M, Wong DW, et al. Serous detachment of the retina. Retina. 2003; 23:820–846.
3. Guyer DR, Yannuzzi LA, Slakter JS, et al. Digital indocyanine green videoangiography of central serous chorioretinopathy. Arch Ophthalmol. 1994; 112:1057–1062.
4. Hee MR, Puliafito CA, Wong C, et al. Optical coherence tomography of central serous chorioretinopathy. Am J Ophthalmol. 1995; 120:65–74.
5. Gomez-Ulla F, Vazquez JM, Rodriguez-Cid MJ, et al. Central serous chorioretinopathy following pigment epithelium detachment: fluorescein and indocyanine green angiography follow-up. Acta Ophthalmol Scand. 2000; 78:232–234.
6. Imamura Y, Fujiwara T, Margolis R, Spaide RF. Enhanced depth imaging optical coherence tomography of the choroid in central serous chorioretinopathy. Retina. 2009; 29:1469–1473.
7. Nakayama M, Keino H, Okada AA, et al. Enhanced depth imaging optical coherence tomography of the choroid in Vogt-Koyanagi-Harada disease. Retina. 2012; 32:2061–2069.
8. Fardeau C, Tran TH, Gharbi B, et al. Retinal fluorescein and indocyanine green angiography and optical coherence tomography in successive stages of Vogt-Koyanagi-Harada disease. Int Ophthalmol. 2007; 27:163–172.
9. Yang P, Ren Y, Li B, et al. Clinical characteristics of Vogt-Koyanagi-Harada syndrome in Chinese patients. Ophthalmology. 2007; 114:606–614.
10. Kunavisarut P, Pathanapitoon K, van Schooneveld M, Rothova A. Chronic central serous chorioretinopathy associated with serous retinal detachment in a series of Asian patients. Ocul Immunol Inflamm. 2009; 17:269–277.
11. Kang JE, Kim HJ, Boo HD, et al. Surgical management of bilateral exudative retinal detachment associated with central serous chorioretinopathy. Korean J Ophthalmol. 2006; 20:131–138.
12. Bouchenaki N, Morisod L, Herbort CP. Vogt-Koyanagi-Harada syndrome: importance of rapid diagnosis and therapeutic intervention. Klin Monbl Augenheilkd. 2000; 216:290–294.
13. Yannuzzi LA. Central serous chorioretinopathy: a personal perspective. Am J Ophthalmol. 2010; 149:361–363.
14. Wang MS, Sander B, Larsen M. Retinal atrophy in idiopathic central serous chorioretinopathy. Am J Ophthalmol. 2002; 133:787–793.
15. Ross A, Ross AH, Mohamed Q. Review and update of central serous chorioretinopathy. Curr Opin Ophthalmol. 2011; 22:166–173.
16. Read RW, Holland GN, Rao NA, et al. Revised diagnostic criteria for Vogt-Koyanagi-Harada disease: report of an international committee on nomenclature. Am J Ophthalmol. 2001; 131:647–652.
17. Levine R, Brucker AJ, Robinson F. Long-term follow-up of idiopathic central serous chorioretinopathy by fluorescein angiography. Ophthalmology. 1989; 96:854–859.
18. Guyer DR, Puliafito CA, Mones JM, et al. Digital indocyanine-green angiography in chorioretinal disorders. Ophthalmology. 1992; 99:287–291.
19. Herbort CP, Mantovani A, Bouchenaki N. Indocyanine green angiography in Vogt-Koyanagi-Harada disease: angiographic signs and utility in patient follow-up. Int Ophthalmol. 2007; 27:173–182.
20. Miyanaga M, Kawaguchi T, Miyata K, et al. Indocyanine green angiography findings in initial acute pretreatment Vogt-Koyanagi-Harada disease in Japanese patients. Jpn J Ophthalmol. 2010; 54:377–382.
21. Lin D, Chen W, Zhang G, et al. Comparison of the optical coherence tomographic characters between acute Vogt-Koyanagi-Harada disease and acute central serous chorioretinopathy. BMC Ophthalmol. 2014; 14:87.
22. Kato Y, Yamamoto Y, Tabuchi H, et al. Retinal pigment epithelium folds as a diagnostic finding of Vogt-Koyanagi-Harada disease. Jpn J Ophthalmol. 2013; 57:90–94.
23. Early Treatment Diabetic Retinopathy Study design and baseline patient characteristics: ETDRS report number 7. Ophthalmology. 1991; 98:5 Suppl. 741–756.
24. Spaide RF, Koizumi H, Pozzoni MC. Enhanced depth imaging spectral-domain optical coherence tomography. Am J Ophthalmol. 2008; 146:496–500.
25. Tugal-Tutkun I, Herbort CP, Khairallah M. Scoring of dual fluorescein and ICG inflammatory angiographic signs for the grading of posterior segment inflammation (dual fluorescein and ICG angiographic scoring system for uveitis). Int Ophthalmol. 2010; 30:539–552.
26. Pryds A, Sander B, Larsen M. Characterization of subretinal fluid leakage in central serous chorioretinopathy. Invest Ophthalmol Vis Sci. 2010; 51:5853–5857.
27. Lee JE, Park SW, Lee JK, et al. Edema of the photoreceptor layer in Vogt-Koyanagi-Harada disease observed using high-resolution optical coherence tomography. Korean J Ophthalmol. 2009; 23:74–79.
28. Hooymans JM. Fibrotic scar formation in central serous chorioretinopathy developed during systemic treatment with corticosteroids. Graefes Arch Clin Exp Ophthalmol. 1998; 236:876–879.
29. Loo JL, Lee SY, Ang CL. Can long-term corticosteriods lead to blindness? A case series of central serous chorioretinopathy induced by corticosteroids. Ann Acad Med Singapore. 2006; 35:496–499.
30. Harikrishnan A, Anderson K, Patra S. Severe visual loss secondary to central serous chorioretinopathy following prolonged immune suppression with oral prednisolone. JRSM Short Rep. 2010; 1:31.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download