Abstract
Purpose
To report the frequency and clinical features of sterile inflammation after intravitreal aflibercept injection in a Korean population.
Methods
A single-center, retrospective study was performed in patients who received intravitreal aflibercept from July 2013 through January 2015.
Results
A total of four cases of post-injection sterile inflammation were identified from 723 aflibercept injections in 233 patients. Patients presented 1 to 13 days after intravitreal aflibercept injection (mean, 5 days). The mean baseline visual acuity was 20 / 60, which decreased to 20 / 112 at diagnosis but ultimately recovered to 20 / 60. Three cases had inflammatory cells in the anterior chamber (mean, 2.25+; range, 0 to 4+), and all cases had vitritis (mean, 3+; range, 2+ to 4+). No patients had pain. Only one patient underwent anterior chamber sampling (culture negative) and injection of antibiotics. Three of four patients were treated with a topical steroid, and all experienced improvement in their symptoms and signs of inflammation.
Conclusions
The overall incidence of sterile inflammation after intravitreal aflibercept injection in a Korean population was 4 of 723 injections (0.55%), or 4 of 233 patients (1.79%). Sterile inflammation after intravitreal aflibercept injection typically presents without pain, and the visual outcomes are generally favorable.
The intravitreal injection of anti-vascular endothelial growth factor (VEGF) agents is remarkably effective for many retinal diseases, including neovascular age-related macular degeneration (AMD), macular edema following retinal vein occlusion, and diabetic macular edema [12345678].
Recently, a new anti-VEGF agent, aflibercept (Eylea; Regeneron, Tarrytown, NY, USA), was introduced for treatment of several retinal diseases. Aflibercept is a soluble decoy receptor fusion protein, consisting of portions of two VEGF receptors, VEGFR-1 and VEGFR-2, which binds to all isoforms of VEGF-A, as well as placental growth factor, and blocks their activity [9]. Many studies have shown that aflibercept is effective for neovascular AMD, retinal vein occlusion, and diabetic macular edema [910111213].
However, several case series have reported sterile inflammation after intravitreal aflibercept injections in the United States [141516]. To our knowledge, there are no reports about sterile inflammation after aflibercept injection in Korea. The purpose of this retrospective case series was to describe the frequency, clinical characteristics, and treatment of sterile inflammation after intravitreal aflibercept injection in a Korean population.
This study adhered to the ethical standards in the Declaration of Helsinki. The medical records of all patients treated by the retina specialists at Nune Eye Hospital (Seoul, Korea) who developed sterile inflammation after an intravitreal aflibercept injection between July 18, 2013 and January 19, 2015 were retrospectively reviewed.
All intravitreal injections of aflibercept were performed under aseptic conditions in the operating room. After topical anesthesia using proparacaine (Alcaine; Alcon, Fort Worth, TX, USA), the periocular skin, eyelids, and eyelashes were disinfected with 10% povidone-iodine swabs, and a 5% povidone-iodine ophthalmic solution was applied to the ocular surface. The intravitreal injection was performed using a 30-gauge needle 3.0 to 3.5 mm posterior to the limbus, depending on the patient's phakic status. Patients received a 2 mg/0.05 mL intravitreal aflibercept injection. After intravitreal injection, moxifloxacin ophthalmic solution (Vigamox, Alcon) and topical steroid eyedrops (prednisolone acetate 1% or flourometholone) were administered four times a day for one week.
We collected the pre-injection data including the age and sex of patients, the affected eye, phakic status, the number of prior aflibercept and other anti-VEGF injections, the underlying diagnosis, the pre-injection best-corrected visual acuity (BCVA), and the pre-injection intraocular pressure (IOP). Clinical presentation data were collected including the date of presentation, BCVA, IOP, presence or absence of pain, presenting symptoms and signs, and initial management. The clinical course, including the duration of recovery and the BCVA over time, was collected.
Snellen visual acuities were converted into the logarithm of the minimal angle of resolution (logMAR) for statistical analysis. A Wilcoxon signed ranked test was performed to compare visual acuity between the baseline and post-injection time points. Statistical analyses were performed using SPSS ver. 18.0 (SPSS Inc., Chicago, IL, USA). Statistical significance was achieved when the p-value was less than 0.05.
During the study period, a total of four cases of post-injection sterile inflammation were identified from 723 aflibercept injections in 233 patients. Therefore, the incidence of sterile inflammation after intravitreal aflibercept injection was 0.55% of injections, or 1.79% of patients. The cases were identified from patients of two retina specialists of Nune Eye Hospital (total seven retina specialists). Cases 1 to 3 were treated by OW Kwon and case 4 was treated by YS You.
The mean age at time of presentation with sterile inflammation after aflibercept injection was 73 years (range, 53 to 86 years). All patients were male; three of the affected eyes were on the right side and one was on the left side. There was one phakic eye and three pseudophakic eyes. The mean number of previous aflibercept injections was 5.75 (range, 3 to 10). No patient had a history of uveitis or inflammation after intravitreal injection with another anti-VEGF agent (bevacizumab and/or ranibizumab). Two cases were diagnosed as choroidal neovascularization (CNV), while the other two were diagnosed as polypoidal choroidal vasculopathy (PCV).
In these cases, all patients complained of blurred vision without pain. The inflammation reaction was identified a mean of 5 days (range, 1 to 13 days) after the aflibercept injection. The mean pre-injection BCVA was 0.48 logMAR (range, 0.1 to 1.4). This changed to 0.75 logMAR (range, 0.1 to 1.7) after the injection, but this change was not significant (p = 0.109). The mean pre-injection IOP was 14.25 mmHg (range, 8 to 19 mmHg). This changed to 16.75 mmHg (range, 7 to 26 mmHg) after the injection, but this change was also not significant (p = 0.465). There was one case (case 3) with an IOP >20 mmHg. Anterior inflammation was noted in three cases. Anterior chamber cells measured 0 to 4+ (mean, 2.25+). All cases presented with vitreous inflammation. Vitreous cells measured 2+ to 4+ (mean, 3+). Hypopyon and ciliary injection were not noted in any cases. Aqueous humor sampling and intravitreal injection of antibiotics were performed in one of four cases (case 1), but the culture result was negative.
Three of these patients were treated with prednisolone acetate 1% eye drops (Predforte, Alcon) or flourometholone eye drops (Fumelon; Hanlim, Seoul, Korea). All cases regained their pre-injection BCVA at a mean of 20.75 days (range, 10 to 33 days) after the onset of inflammation. All cases received at least one additional aflibercept injection. The mean interval of retreatment was 5.5 weeks (range, 4 to 8 weeks). No cases retreated with aflibercept showed evidence of recurrent inflammation. The specifics of these data are summarized in Table 1.
A 68-year-old man with a history of CNV secondary to AMD treated with bevacizumab (right eye, OD) ×50, ranibizumab (OD) ×22, and aflibercept (OD) ×7 complained of blurred vision without pain two days after his eighth intravitreal aflibercept injection (OD). The patient's baseline BCVA was 0.63 (Snellen visual acuity), which then dropped to 0.125 after the aflibercept injection. On presentation, there were 4+ anterior chamber cells and 3+ vitreal cells in his right eye (Fig. 1A). An inflammatory strand in the anterior chamber was identified (Fig. 1B). The fundus showed haziness due to vitritis (Fig. 1C). We thought this presentation might be caused by post-intravitreal injection fungal endophthalmitis and performed aqueous humor sampling and administered an intravitreal injection of antibiotics (vancomycin 1.0 mg/0.1 mL, ceftazidime 2.25 mg/0.1 mL, and voriconazole 0.1 mg/0.1 mL) three days after the aflibercept injection. The patient was treated with prednisolone acetate 1% eye drops (Predforte) every six hours, moxifloxacin eye drops (Vigmox) every two hours, and voriconazole tablets (Vfend 200 mg; Pfizer, New York, NY, USA) 400 mg twice daily. The following day, the patient's BCVA improved to 0.2, the anterior chamber cells decreased to 2+, and the vitreous haziness decreased. The results of aqueous humor sampling (smear and culture) were negative, and treatment with voriconazole tablets was discontinued. The symptoms and signs of inflammation had gradually improved. Thirty-five days after the aflibercept injection, the anterior chamber and vitreous were clear (Fig. 1D), and BCVA improved to 0.63. Finally, we diagnosed this case as sterile inflammation after aflibercept injection. The patient received two additional aflibercept injections (OD, ninth and tenth injections), and showed no post-injection inflammation.
An 84-year-old man with a history of glaucoma and PCV treated with bevacizumab (left eye, OS) ×21, ranibizumab (OS) ×18, and aflibercept (OS) ×10 complained of blurred vision without pain four days after his eleventh intravitreal aflibercept injection (OS). The patient's baseline BCVA was 0.04, which dropped to 0.02 after the injection. On presentation, the patient had 3+ anterior chamber cells and 2+ vitreal cells. The fundus showed vitreous floaters at the inferior equator. We thought this case might be due to sterile inflammation. The patient was treated with flourometholone eye drops every two hours and prednisolone (Solondo; Yuhan, Seoul, Korea) 20 mg orally daily, instead of prednisolone acetate eye drops, because he had been treated for glaucoma and had a history of steroid response (in particular, prednisolone acetate 1% eye drops). Fourteen days after the aflibercept injection, the anterior chamber and vitreous were clear, and the patient's BCVA improved to 0.04. The patient received two additional aflibercept injections (OD, twelfth and thirteenth injections), and showed no post-injection inflammation.
When a 53-year-old man with a history of CNV secondary to AMD treated with bevacizumab (OD) ×1, ranibizumab (OD) ×3, and aflibercept ×3 came to the Nune Eye Hospital for his next-day follow-up after the fourth aflibercept injection (OD), post-injection inflammation was identif ied. The patient's baseline BCVA was 0.6, which dropped to 0.5 after the injection. On presentation, he had 2+ anterior chamber cells and 3+ vitreal cells. Vitreous opacity was also identified. Baseline IOP was 16 mmHg (OD), which elevated to 26 mmHg (OD). The patient did not complain of ocular pain. We diagnosed this case as sterile inflammation with elevated IOP and treated the patient with prednisolone acetate 1% eye drops every two hours and dorzolamide 2%/timolol 0.5% eye drops (Lowiop, Hanlim) twice daily. On the eleventh day after the fourth aflibercept injection (OD), the signs and symptoms of inflammation had disappeared, IOP had decreased to 13 mmHg, and the patient's BCVA improved to 0.6. The patient received one additional aflibercept injection (OD, fifth injection) without incident.
An 86-year-old man with a history of PCV treated with bevacizumab (OD) ×1, ranibizumab (OD) ×9, and aflibercept (OD) ×3 complained of a floater (OD) without pain 13 days after his fourth aflibercept injection (OD). The patient's baseline BCVA was 0.1, and this did not change. He had 4+ vitreal cells, but there were no anterior chamber cells. Vitreous opacity was identified on fundus exam. There was no retinal tear on the fundus exam. The patient was observed with topical steroid treatment. Forty-three days after the fourth aflibercept injection, the signs and symptoms of vitritis spontaneously disappeared. We thought this case might be due to sterile inflammation. The patient received one additional aflibercept injection (OD, fifth injection) without incident.
Sterile inflammation after intravitreal aflibercept injection was first reported by Hahn et al. [14] in a nonconsecutive series of 15 cases gathered by the Therapeutic Surveillance Subcommittee of the American Society of Retina Specialists. All but one patient experienced symptoms within three days of the injection. In this case series, 9 of 15 cases (60%) presented with pain, and 6 cases (40%) presented with redness. Visual acuity had generally recovered to baseline levels within one month.
Goldberg et al. [16] reported on the frequency and clinical characteristics of sterile inflammation after intravitreal aflibercept injections. A total of 20 cases of post-injection sterile inflammation were identified in 5,356 aflibercept injections (overall incidence: 0.37% per injection). The patients presented 1 to 13 days after their aflibercept injections, and all noted decreased visual acuity. Only 3 cases had pain (15%), and 2 cases had conjunctival injections (10%). All patients were managed with frequent topical steroids, and all except one patient regained their baseline visual acuity.
Fine et al. [15] published a report on 28 cases of sterile inflammation after a total of 5,905 aflibercept injections (overall incidence: 0.47% per injection). The inflammation was identified 1 to 40 days after the injection. The mean visual acuity dropped at presentation but almost fully recovered after the first month. Pain was identified in 11 of the 28 cases (39%). Visual acuity and inflammation returned to baseline within one month with topical steroid treatment in most cases.
This case series showed the incidence and characteristics of sterile inflammation in four eyes after 723 aflibercept injections in 233 Korean patients. The overall incidence of sterile inflammation was 0.55% of injections, or 1.79% of patients. This incidence rate is a little higher than previous studies of sterile inflammation after aflibercept injections, but the difference is not significant. There were no cases with ocular pain in this study. While this result is inconsistent with previous studies, the difference is likely because our study group was too small. Interestingly, case 4 was not treated with a topical steroid, and the patient improved spontaneously. In this study, the inflammation was identified at 1 to 7 days (mean, 3.5 days) after the aflibercept injection and improved within 10 to 33 days (mean, 20.75 days). This result was consistent with previous studies. In the present study, all cases received at least one additional aflibercept injection after the inflammation resolved, and there were no cases with recurrent post-injection inflammation. Similarly, recurrent post-injection inflammation was found to be uncommon in previous studies [1516].
Inflammatory reactions have been reported after intravitreal injections with two other commonly used anti-VEGF agents, ranibizumab and bevacizumab [171819]. In phase 3 trials (MARINA and ANCHOR) of ranibizumab monthly injections for AMD, the incidence of uveitis with endophthalmitis was 0.05% of injections [12]. In several retrospective studies, the incidence of sterile endophthalmitis has been found to be between 0.09% and 1.1% of bevacizumab injections [202122]. In the phase 3 comparison study of ranibizumab and bevacizumab for neovascular AMD (CATT trial), the incidence of pseudo-endophthalmitis was 0.17% of both bevacizumab and ranibizumab injections [5].
The mechanism of sterile inflammation after the injection of an anti-VEGF agent has not yet been determined; however, a variety of theories have been advanced to explain sterile inflammation. One such theory focuses on a problem with the vehicle formulation. In the FOCUS study of ranibizumab, the incidence of presumed endophthalmitis was 5.7% of patients [23]. The manufacturer switched from a lyophilized formulation to a liquid formulation, and the incidence of inflammatory reactions decreased. Another theory is increased immunogenicity of the therapeutic agents [24]. The immunogenicity of aflibercept was evaluated in the serum of patients in phase 3 trials and found to be approximately 1% to 3%, so there exists a measurable rate of immune reaction against this agent in humans (Aflibercept, Food and Drug Administration package insert, http://www.regeneron.com/Eylea/eylea-fpi.pdf). Drug impurities due to defects of manufacturing may be another reason for increased immunogenicity. Clustering around specific lot numbers was reported by Hahn et al. [14] and Fine et al. [15] This clustering is suspected to be due to a manufacturing defect. In this retrospective study, we did not check the lot numbers of the administered aflibercept; therefore, we are unable to confirm a clustering outbreak.
This study has several limitations. It was a small, retrospective case series. Therefore, we could have missed sterile inflammation cases after aflibercept injections due to physician omission and non-detection. These errors could lead to an underestimated incidence rate. Moreover, these cases had not been treated by a defined clinical protocol. Because of the different treatment for each patient, there may be an error in the analysis of the outcomes. Finally, case 1 was treated with an intravitreal antibiotic injection and systemic antifungal agent. Culture-negative infectious endophthalmitis cannot be ruled out as the cause of symptoms in this case.
In conclusion, the overall incidence of sterile inflammation after intravitreal aflibercept injections in a Korean population was 4 of 723 injections (0.55%), or 4 of 233 patients (1.79%). Sterile inflammation after intravitreal aflibercept injection typically presents without pain, and visual outcomes are generally favorable. We also found that aflibercept retreatment after recovery from sterile inflammation was generally safe. Further study is needed to investigate the causes of sterile inflammation after aflibercept injections.
Figures and Tables
Fig. 1
Fundus photo images and a slit lamp photo image of a 69-year-old male patient (case 1). (A) Baseline fundus photo image, taken prior to the eighth intravitreal aflibercept injection. (B) Slit-lamp photo image. (C) Fundus photo image on the day of presentation. (D) Fundus photo image after resolution of inflammation.
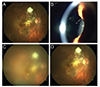
Table 1
Summary of case characteristics

IAI = intravitreal aflibercept injection; VA = visual acuity; Pres VA = visual acuity on the day of presentation; IOP = intraocular pressure; A/C = anterior chamber; Vit = vitreous; VA return = time (in days) for return of vision to baseline on the day of IAI; OD = right eye; Pseudo = pseudophakia; OS = left eye.
References
1. Brown DM, Kaiser PK, Michels M, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006; 355:1432–1444.
2. Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006; 355:1419–1431.
3. Brown DM, Campochiaro PA, Singh RP, et al. Ranibizumab for macular edema following central retinal vein occlusion: six-month primary end point results of a phase III study. Ophthalmology. 2010; 117:1124–1133.e1.
4. Campochiaro PA, Heier JS, Feiner L, et al. Ranibizumab for macular edema following branch retinal vein occlusion: six-month primary end point results of a phase III study. Ophthalmology. 2010; 117:1102–1112.e1.
5. CATT Research Group. Martin DF, Maguire MG, et al. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med. 2011; 364:1897–1908.
6. Heier JS, Campochiaro PA, Yau L, et al. Ranibizumab for macular edema due to retinal vein occlusions: long-term follow-up in the HORIZON trial. Ophthalmology. 2012; 119:802–809.
7. Brown DM, Nguyen QD, Marcus DM, et al. Long-term outcomes of ranibizumab therapy for diabetic macular edema: the 36-month results from two phase III trials: RISE and RIDE. Ophthalmology. 2013; 120:2013–2022.
8. Schmidt-Erfurth U, Lang GE, Holz FG, et al. Three-year outcomes of individualized ranibizumab treatment in patients with diabetic macular edema: the RESTORE extension study. Ophthalmology. 2014; 121:1045–1053.
9. Heier JS, Brown DM, Chong V, et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012; 119:2537–2548.
10. Korobelnik JF, Do DV, Schmidt-Erfurth U, et al. Intravitreal aflibercept for diabetic macular edema. Ophthalmology. 2014; 121:2247–2254.
11. Korobelnik JF, Holz FG, Roider J, et al. Intravitreal aflibercept injection for macular edema resulting from central retinal vein occlusion: one-year results of the phase 3 GALILEO Study. Ophthalmology. 2014; 121:202–208.
12. Schmidt-Erfurth U, Kaiser PK, Korobelnik JF, et al. Intravitreal af libercept injection for neovascular age-related macular degeneration: ninety-six-week results of the VIEW studies. Ophthalmology. 2014; 121:193–201.
13. Campochiaro PA, Clark WL, Boyer DS, et al. Intravitreal aflibercept for macular edema following branch retinal vein occlusion: the 24-week results of the VIBRANT study. Ophthalmology. 2015; 122:538–544.
14. Hahn P, Kim JE, Stinnett S, et al. Aflibercept-related sterile inflammation. Ophthalmology. 2013; 120:1100–1101.e1-5.
15. Fine HF, Roth DB, Shah SP, et al. Frequency and characteristics of intraocular inflammation after aflibercept injection. Retina. 2015; 35:681–686.
16. Goldberg RA, Shah CP, Wiegand TW, Heier JS. Noninfectious inflammation after intravitreal injection of aflibercept: clinical characteristics and visual outcomes. Am J Ophthalmol. 2014; 158:733–737.
17. Bakri SJ, Larson TA, Edwards AO. Intraocular inflammation following intravitreal injection of bevacizumab. Graefes Arch Clin Exp Ophthalmol. 2008; 246:779–781.
18. Fauser S, Schroeder S, Caramoy A, et al. Intraocular inflammation after intravitreal ranibizumab injections. Acta Ophthalmol. 2011; 89:e98–e99.
19. Kay CN, Tarantola RM, Gehrs KM, et al. Uveitis following intravitreal bevacizumab: a non-infectious cluster. Ophthalmic Surg Lasers Imaging. 2011; 42:292–296.
20. Wickremasinghe SS, Michalova K, Gilhotra J, et al. Acute intraocular inflammation after intravitreous injections of bevacizumab for treatment of neovascular age-related macular degeneration. Ophthalmology. 2008; 115:1911–1915.
21. Wu L, Martinez-Castellanos MA, Quiroz-Mercado H, et al. Twelve-month safety of intravitreal injections of bevacizumab (Avastin): results of the Pan-American Collaborative Retina Study Group (PACORES). Graefes Arch Clin Exp Ophthalmol. 2008; 246:81–87.
22. Georgopoulos M, Polak K, Prager F, et al. Characteristics of severe intraocular inflammation following intravitreal injection of bevacizumab (Avastin). Br J Ophthalmol. 2009; 93:457–462.
23. Antoszyk AN, Tuomi L, Chung CY, et al. Ranibizumab combined with verteporfin photodynamic therapy in neovascular age-related macular degeneration (FOCUS): year 2 results. Am J Ophthalmol. 2008; 145:862–874.
24. Marticorena J, Romano V, Gomez-Ulla F. Sterile endophthalmitis after intravitreal injections. Mediators Inflamm. 2012; 2012:928123.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download