Abstract
Purpose
To evaluate the factors affecting treatment outcome of graft infection following penetrating keratoplasty (PKP).
Methods
In this retrospective study, 28 patients who underwent PKP between January 2005 and January 2013 and who were diagnosed with graft infection were classified into a treatment success group or a treatment failure group. Demographic and clinical characteristics, as well as the results of the microbiologic investigation, were analyzed and compared. A subsequent binary logistic regression analysis was performed to identify the prognostic factors affecting treatment outcome.
Results
Graft infection occurred at a mean of 38.29 ± 36.16 months (range, 1 to 96 months) after PKP. Seventeen patients developed bacterial keratitis, and 11 patients developed fungal keratitis. Overall, of the 28 patients, nine (32.1%) were classified in the treatment failure group. Multivariate analysis identified pre-existing graft failure (p = 0.019), interval longer than 72 hours between donor death and PKP (p = 0.010), and fungal infection (p = 0.026) as significant risk factors for treatment failure.
The cornea has a mechanism of protecting itself against bacterial, viral, or fungal infections, but this mechanism often weakens after penetrating keratoplasty (PKP) procedures. Moreover, other factors, such as long-term use of topical corticosteroid and soft contact lens, graft hypoesthesia, suture-related problems, and ocular surface disorders, including dry eye, make the cornea more vulnerable to infection [12345]. The incidence of graft infection following PKP varies from 1.76% to 12.1% [1234567].
Despite improvement in antibiotic treatment, the treatment failure of microbial keratitis remains an important problem. The rate of treatment failure is estimated to be around 10% [89], but it is significantly higher in eyes that underwent PKP, ranging from 26% to 57%, [567]. Some studies have reported that long-term prophylactic antibiotic administration is a risk factor for the emergence of antibiotic-resistant flora, and this may be related to the poor treatment outcome of graft infection [1011].
Graft infection is a serious complication of PKP and can lead to poor visual outcomes. Hence, knowing the factors that affect treatment outcome can improve patient prognosis. Although several studies have evaluated the risk factors of graft infection, to the best of our knowledge, there have been no reports on the prognostic factors affecting treatment outcome [12345]. Therefore, we performed a comparative study between "treatment success" and "treatment failure" groups among patients who showed graft infection following PKP and investigated the factors associated with poor treatment outcome.
A retrospective chart review of medical records was performed on 323 eyes of 323 consecutive patients who underwent PKP at Chonnam National University Hospital between January 2005 and January 2013 and who were followed-up for at least one year postoperatively. All study protocols adhered to the tenets of the Declaration of Helsinki and were approved by the institutional review board of Chonnam National University Hospital. We only included eyes with smear- or culture-proven infectious keratitis (non-viral). Patients with recurrent infectious keratitis caused by a pre-PKP infection were excluded.
The PKP procedure was performed by a single experienced surgeon (KCY) under either general or retrobulbar anesthesia. All donor corneas were preserved in Optisol GS (Chiron Ophthalmics, Irvine, CA, USA). After transplantation, all patients were treated with topical 1% prednisolone acetate and topical 1% cyclosporine A. The initial topical corticosteroid regimen was one drop hourly during postoperative week 1 in patients with vascularized corneas and one drop every two hours in all other patients. Corticosteroid use was tapered gradually over a period of one year and continued until all sutures were removed. In high-risk eyes with vascularized corneas and history of rejection episodes, the lowest steroid regimen permitting absence of rejection episodes was determined, and topical corticosteroid use was continued. Topical 1% cyclosporine A was administered every two hours during postoperative week 1 and tapered over a period of a few months. Patients without contraindication regarding the application of systemic immunosuppresants were also started on oral prednisolone 1 mg/kg and cyclosporine A 5 mg/kg daily and tapered postoperatively over one month. In high-risk cases, application of systemic steroids was prolonged at the discretion of the surgeon. Oral steroid and cyclosporine A were discontinued when local or systemic side effects developed. Suture removal was performed step-by-step over two to three times within one year after surgery.
Smear and culture analyses were performed for all cases showing corneal infiltrate and overlying epithelial defects. Corneal scrapings were obtained from the peripheral and central areas of the corneal infiltration. Gram staining and potassium hydroxide (KOH) wet mount were routinely performed for all smears. Routine culture media included blood agar, chocolate agar, MacConkey agar, and Sabouraud agar. Blood and chocolate agar plates were stored in carbon dioxide environments, whereas MacConkey and Sabouraud plates were stored in oxygen environments. A second blood agar plate was stored in an anaerobic environment. Blood agar, chocolate agar, and MacConkey agar plates were stored at 35℃, whereas separate Sabouraud agar plates were stored at 25℃ and 35℃. The minimum criterion for a positive culture was the growth of three colonies on one solid medium [12]. Routine antibiotic sensitivity testing was performed on all isolates.
The protocol for initial therapy consisted of admission of all cases of graft infection and instillation of topical gatifloxacin or moxifloxacin eye drops. On the basis of the results of the corneal scraping evaluation, patients with presumed gram-positive microbial keratitis were additionally administered topical fortified cefazolin (50 mg/mL) or vancomycin (25 mg/mL), and those with gram-negative microbial keratitis were given topical tobramycin (14 mg/mL) or ceftazidime (50 mg/mL). Patients with fungal keratitis were initially started on topical amphotericin 0.15% or voriconazole 1% and oral fluconazole. Eye drops were administered every hour during the first 48 hours, and subsequent modification of treatment was based upon the results of cultures and antibiotic sensitivities or clinical response. Topical corticosteroid was suspended in all cases at the time of presentation.
We recorded information on age, sex, primary diagnosis before PKP, interval between PKP and graft infection, graft state before onset of infection (failed or clear graft), donor data (age and death to surgery duration), duration of symptoms, medical history before graft infection, and slit lamp findings at initial presentation. The size of the corneal infiltration was measured in two dimensions using the graduated slit beam, and the presence of hypopyon was noted. The results of the microbiologic investigation were also recorded. Graft failure was defined as an irreversible loss of central graft clarity from any cause.
Treatment success was defined as resolution of the infiltration and complete healing of the epithelial defects. Infectious keratitis was considered to have failed treatment if the size of infiltration remained the same or increased or if the ulcer perforated and required surgical intervention (corneal gluing, graft resuturing, re-PKP, or evisceration).
The putative prognostic factors for treatment outcome were compared between the two groups (treatment success versus treatment failure) with a Mann-Whitney U-test for continuous variables and a chi-squared test or Fisher's exact test for categorical variables. A subsequent logistic regression analysis was performed to identify any independent associations between the prognostic factors and treatment outcome, and odds ratio and their 95% confidence intervals were estimated. All statistical analyses were performed using the PASW Statistics ver. 18.0 (SPSS Inc., Chicago, IL, USA). A p-value less than 0.05 was considered statistically significant.
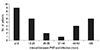
The incidence of graft infection was 10.5% (34 of 323 eyes). Among these 34 eyes, five showed smear- and culture-negative keratitis and one case was lost to follow-up. Thus, 28 eyes of 28 patients were included in the analysis. Infection occurred at a mean of 38.29 ± 36.16 months (range, 1 to 96 months) after PKP. Of the 28 patients, nine (32.1%) developed graft infection within one year of PKP; among these nine patients, seven developed an infection within six months of PKP. The remaining 19 (67.9 %) patients developed graft infection one year or more after PKP. The distribution of duration between PKP and graft infection is shown in Fig. 1.
The mean age at diagnosis of graft infection was 64.82 ± 14.06 years (range, 35 to 85 years). The main indications for PKP were infectious corneal ulcer (9 / 28, 32.1%) and pseudophakic bullous keratopathy (7 / 28, 25.0%). Seven patients (25.0%) had a pre-existing graft failure, and 21 (75.0%) had clear grafts at the time of presentation. The mean duration of symptoms before hospital presentation was 6.73 ± 4.23 days (range, 1 to 16 days). The proportion of patients receiving topical antibiotics, topical corticosteroids, and systemic corticosteroids at the time of onset of graft infection was 17.9%, 78.6%, and 32.1%, respectively (Table 1).
Seventeen of the 28 patients had positive results in Gram staining, and the remaining 11 patients showed smear-positive results for fungus on KOH wet mount. Microbiological culture yielded positive results in 15 cases (53.6%). The most common bacterial species isolated was Streptococcus species (6 / 15, 40.0%). Fungal infections were most commonly caused by Candida species (2 / 15, 13.3%) and Fusarium species (2 / 15, 13.3%). There was no patient with a polymicrobial infection. Details of the isolated organisms are detailed in Table 2.
Overall, 19 of the 28 patients (67.9%) were classified in the treatment success group. All patients classified in the treatment failure group (n = 9) required an adjunctive surgical procedure: five underwent re-PKP, two required evisceration, and the remaining two underwent corneal gluing. There was no statistically significant difference in age, sex, primary diagnosis, interval between PKP and graft infection, age of the donor cornea, size of the corneal infiltration, or rate of positive hypopyon and positive culture between the two groups. Regarding the medical history at the time of onset of the graft infection, a significantly higher proportion of patients in the treatment failure group used systemic corticosteroids (66.7% vs. 18.8% in the treatment success group; p = 0.013). Pre-existing graft failure (55.6%) and fungal keratitis (66.7%) were more predominant in the treatment failure group (p = 0.020 and p = 0.035, respectively). Mean interval between donor death and PKP was significantly longer in the treatment failure group (108.50 ± 62.22 hours) compared with the treatment success group (46.18 ± 39.44 hours, p = 0.005). Mean interval between the onset of symptoms and hospital presentation was also significantly longer in the treatment failure group (9.33 ± 4.47 vs. 5.58 ± 3.19 days, p = 0.038) (Table 3).
Table 4 shows the results of the multivariate logistic regression analysis, which identified pre-existing graft fail ure (p = 0.019), interval longer than 72 hours between donor death and PKP (p = 0.010), and fungal infection (p = 0.026) as the significant risk factors of treatment failure.
In this study, we investigated the association of various factors with treatment outcome of graft infection. We found that pre-existing graft failure, extended preservation time, and fungal keratitis were associated with treatment failure.
In the present study, the incidence of graft infection after PKP was 10.5%. Considering that our study excluded patients with recurrent infection, this number is slightly higher than those reported in the literature [351314]. It should be noted that a considerable number of patients in our study were agricultural workers, which has been shown to be an important risk factor for graft infection [15]. This characteristic might also explain the significantly high incidence of fungal graft infections (39.3%) [1617].
Graft infection is known to occur after PKP. The time course for development of graft infection following PKP seems to be related to the major contributing factor for infection, which are suture complication and trauma or other risk factors such as ocular surface disease. Previous studies have reported that most graft infections often occur within one year of PKP [7131819]. However, in the current study, the majority (67.9%) of infections occurred after the one-year postoperative period. The high incidence rates of ocular trauma in agricultural work might have contributed to the late onset of graft infection. Suture-related infection, which is also known to be closely related to late-onset graft infection, was seen in only three of the 19 patients (10.7%), whereas seven patients had a history of minor ocular trauma involving contact with organic matter. If all cases of presumed graft infection were included in the analysis, the estimated incidence of suture-related infection would be 8.8%. Considering that suture-related problems, such as loose or broken sutures, are major risk factors for the development of microbial keratitis following PKP, we observed a lower incidence rate compared to the findings reported previously [123456710131920]. This might be explained by frequent follow-ups and aggressive suture management in the first year after surgery, including step-by step removal of the corneal sutures more than two to three times within one year after PKP.
The rate of positive microbial cultures in our study was lower than that reported in previous studies [12345671011131420]. The choice of medium or agar plates instead of brain heart infusion culture medium (BHI) to inoculate the corneal sample could offer a possible explanation for our low isolation rate [20]. In addition, since the study was conducted in a tertiary care center, many cases were referred for evaluation after topical antibiotic treatment had already started, affecting the rate of positive cultures. As expected, the majority of the organisms were Gram-positive and Streptococcus species (40.0% of isolates).
Despite aggressive medical treatment, infectious keratitis increases the risk for endophthalmitis, corneal thinning, descemetocele formation, and, ultimately, corneal perforation, which is associated with an unfavorable prognosis for visual acuity. Although the treatment failure rate varies between reports, it is known to be higher in eyes that undergo PKP [567]. In this study, surgical interventions were required in nine (32.1%) patients. In a comparative analysis of the treatment failure and treatment success groups, there were significantly higher rates of pre-existing graft failure, systemic corticosteroid use, and fungal keratitis in the treatment failure group. The interval between donor death and PKP and the mean interval between the onset of symptoms and diagnosis were longer in the treatment failure group than in the treatment success group. However, logistic regression analysis showed that only pre-existing graft failure, longer interval between donor death and PKP, and fungal keratitis were statistically significant prognostic factors of poor treatment outcome in graft infection (p = 0.019, 0.010, and 0.026, respectively). Although studies have reported increased poor treatment outcome of graft infection in cases involving long-term administration of prophylactic antibiotics, we did not find an association between use of topical antibiotics and unsuccessful treatment [1011].
The present study suggests that graft state is an important prognostic factor pf treatment outcome in graft infection. Pre-existing graft failure was a risk factor for treatment failure, possibly due to inhibition of wound healing or to the nature of the accompanying underlying ocular problems.
Regarding the characteristics of the donor cornea, the interval from donor death to PKP was more than twice as long in the treatment failure group than in the treatment success group; a longer interval was associated with treatment failure in the multivariate analysis. Given that increasing postmortem and storage times have been reported to reduce endothelial cell density in donor corneas, and that endothelial cell density is an important factor affecting graft prognosis, low endothelial function might be attributable to low graft function and poor wound healing and, consequently, poor prognosis in graft infection [212223242526]. However, because of insufficient medical records, it was difficult to determine whether the outcome was more influenced by donor death to processing time or corneal storage time. In addition, lack of data on endothelial cell density is another drawback in the present study. To allow more conclusive findings, additional studies needed.
Our results also demonstrate the unfavorable prognosis of fungal infection. This finding is in accordance with a previous study, in which fungal keratitis was found to be more likely to perforate the cornea than bacterial keratitis and was generally poorly responsive to conventional medical treatment due to the paucity of available antifungal agents and their high costs [27]. In our series, six of 11 patients diagnosed with fungal infection did not experience remission, representing 54.5% of the cases.
Our results should be interpreted in the context of the study's limitations. First, there are many other factors that might affect treatment outcome in graft infection. However, due to the retrospective nature of the study, we could not determine all the parameters related to treatment outcome. Second, the sample size was insufficient. Therefore, the findings of the multivariate analysis should be interpreted with caution, as the limited number of cases could have introduced additional bias. Further studies with larger sample sizes are needed to confirm the findings of our study.
In conclusion, the results of this study suggest that pre-existing graft failure, extended preservation time, and fungal keratitis are significantly associated with treatment failure. Knowing these prognostic factors and predicting the treatment outcome might be helpful in the management of graft infection.
Figures and Tables
Table 2
Results of staining and culture in patients with graft infection following penetrating keratoplasty

Acknowledgements
The study was partially supported by CNUH Biomedical Research Institute (CRI 13906-22) and Forest Science and Technology Projects (no. S121313L050100) provided by the Korea Forest Service.
References
1. Bates AK, Kirkness CM, Ficker LA, et al. Microbial keratitis after penetrating keratoplasty. Eye (Lond). 1990; 4(Pt 1):74–78.
2. Wright TM, Afshari NA. Microbial keratitis following corneal transplantation. Am J Ophthalmol. 2006; 142:1061–1062.
3. Wagoner MD, Al-Swailem SA, Sutphin JE, Zimmerman MB. Bacterial keratitis after penetrating keratoplasty: incidence, microbiological profile, graft survival, and visual outcome. Ophthalmology. 2007; 114:1073–1079.
4. Leahey AB, Avery RL, Gottsch JD, et al. Suture abscesses after penetrating keratoplasty. Cornea. 1993; 12:489–492.
5. Vajpayee RB, Sharma N, Sinha R, et al. Infectious keratitis following keratoplasty. Surv Ophthalmol. 2007; 52:1–12.
6. Das S, Constantinou M, Ong T, Taylor HR. Microbial keratitis following corneal transplantation. Clin Experiment Ophthalmol. 2007; 35:427–431.
7. Akova YA, Onat M, Koc F, et al. Microbial keratitis following penetrating keratoplasty. Ophthalmic Surg Lasers. 1999; 30:449–455.
8. Gudmundsson OG, Ormerod LD, Kenyon KR, et al. Factors influencing predilection and outcome in bacterial keratitis. Cornea. 1989; 8:115–121.
9. McLeod SD, LaBree LD, Tayyanipour R, et al. The importance of initial management in the treatment of severe infectious corneal ulcers. Ophthalmology. 1995; 102:1943–1948.
10. Fong LP, Ormerod LD, Kenyon KR, Foster CS. Microbial keratitis complicating penetrating keratoplasty. Ophthalmology. 1988; 95:1269–1275.
11. Harris DJ Jr, Stulting RD, Waring GO 3rd, Wilson LA. Late bacterial and fungal keratitis after corneal transplantation. Spectrum of pathogens, graft survival, and visual prognosis. Ophthalmology. 1988; 95:1450–1457.
12. Armstrong M. The laboratory investigation of infective keratitis. Br J Biomed Sci. 1994; 51:65–72.
13. Al-Hazzaa SA, Tabbara KF. Bacterial keratitis after penetrating keratoplasty. Ophthalmology. 1988; 95:1504–1508.
14. Constantinou M, Jhanji V, Vajpayee RB. Clinical and microbiological profile of post-penetrating keratoplasty infectious keratitis in failed and clear grafts. Am J Ophthalmol. 2013; 155:233–237.e2.
15. Srinivasan M, Gonzales CA, George C, et al. Epidemiology and aetiological diagnosis of corneal ulceration in Madurai, south India. Br J Ophthalmol. 1997; 81:965–971.
16. Bharathi MJ, Ramakrishnan R, Vasu S, et al. Epidemiological characteristics and laboratory diagnosis of fungal keratitis: a three-year study. Indian J Ophthalmol. 2003; 51:315–321.
17. Xie L, Zhong W, Shi W, Sun S. Spectrum of fungal keratitis in north China. Ophthalmology. 2006; 113:1943–1948.
18. Tavakkoli H, Sugar J. Microbial keratitis following penetrating keratoplasty. Ophthalmic Surg. 1994; 25:356–360.
19. Vajpayee RB, Boral SK, Dada T, et al. Risk factors for graft infection in India: a case-control study. Br J Ophthalmol. 2002; 86:261–265.
20. Moorthy S, Graue E, Jhanji V, et al. Microbial keratitis after penetrating keratoplasty: impact of sutures. Am J Ophthalmol. 2011; 152:189–194.e2.
21. Borderie VM, Scheer S, Touzeau O, et al. Donor organ cultured corneal tissue selection before penetrating keratoplasty. Br J Ophthalmol. 1998; 82:382–388.
22. Doughman DJ, Van Horn D, Harris JE, et al. Endothelium of the human organ cultured cornea: an electron microscopic study. Trans Am Ophthalmol Soc. 1973; 71:304–324.
23. McKinnon JR, Walters GD. Cadaver storage time. An important factor in donor cornea survival. Arch Ophthalmol. 1976; 94:217–220.
24. Hagenah M, Simon B, Bohnke M. Experimental corneal cryopreservation: impact of postmortem time on corneal endothelial cell survival. Ophthalmic Res. 1993; 25:210–215.
25. Pels E, Beele H, Claerhout I. Eye bank issues. II. Preservation techniques: warm versus cold storage. Int Ophthalmol. 2008; 28:155–163.
26. Stocker FW. The endothelium of the cornea and its clinical implications. Trans Am Ophthalmol Soc. 1953; 51:669–786.
27. Lalitha P, Prajna NV, Kabra A, et al. Risk factors for treatment outcome in fungal keratitis. Ophthalmology. 2006; 113:526–530.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download