This article has been
cited by other articles in ScienceCentral.
Abstract
Purpose
To analyze healing changes of corneal wounds of different corneal incision sizes with or without stromal hydration in cataract surgery using anterior segment optical coherence tomography.
Methods
Cataract surgeries were performed by a single surgeon and 2.2- and 2.8-mm corneal incisions were made using a diamond blade (ME-759; Meyco, Biel-Bienne, Swiss). Patients were divided into four groups according to incision size (2.2 and 2.8 mm), and with/without stromal hydration. Fifteen eyes were assigned to each group and incision wounds were measured using anterior segment optical coherence tomography at 2 hours, 1 day, 1 week, 1 month, and 3 months postoperatively. Corneal thickness, incision length and incision angle were measured and existence of epithelial, endothelial gaping and Descemet's membrane detachment was evaluated.
Results
Incision thickness was greater in the group with stromal hydration than in the group without on operation day (p < 0.05). Stromal hydration exerted greater influence in the 2.2-mm incision group than in the 2.8-mm incision group. Corneal thickness decreased more rapidly in the stromal hydration group than in the group with no hydration (p = 0.022). Endothelial gaping was greater in the 2.2-mm incision group than in the 2.8-mm incision group 1 day, 1 month, and 3 months after surgery (p = 0.035, p = 0.009, and p = 0.008, respectively). No other statistical significance was observed between the two groups (2.2 and 2.8 mm) during follow-up regarding corneal thickness, epithelial gaping and Descemet's membrane detachment.
Conclusions
Corneal wounds with a smaller incision could be more vulnerable to external stimuli such as stromal hydration and are less stable than those with a larger incision.
Keywords: Corneal pachymetry, Corneal stroma, Wounds and injuries
Currently, microincision coaxial cataract surgery (MICS) is a state-of-the-art cataract removal treatment with reduced rates of wound leaks, astigmatism and postoperative infection. However, when compared with conventional coaxial cataract surgery, its overall advantage remains questionable. Phacoemulsification of microincisions may require longer and more intense ultrasound exposure, create difficulty in handling, and may inflict greater loss of endothelial cells [
1,
2,
3,
4]. Furthermore, several reports indicate that surgically-induced astigmatism does not differ whether MICS or a conventional approach is used [
1,
2,
3,
4,
5].
Clear corneal incision increases the risk of endophthalmitis, which is countered by corneal stromal hydration. The latter is applied to enhance the wound sealing and to prevent inflow of ocular surface fluid. Nevertheless, the tendency for endothelial misalignment is increased with stromal hydration in the early postoperative period [
6] thus, incisional size and the use of stromal hydration are key variables affecting configuration and healing of clear corneal incisions.
Anterior segment optical coherence tomography (AS-OCT) is a sophisticated imaging technique that allows visualization of the incisions in real time and qualitative analysis of structural changes in the cornea [
6]. In the present study, we determined differences in wound structure and changes in dynamic healing in variably-sized corneal incisions (with and without stromal hydration) using AS-OCT.
Materials and Methods
In this retrospective case study, the clinical records of patients who underwent cataract surgery at our hospital from August, 2012 to December, 2013 were reviewed.
A total of 60 eyes undergoing standard cataract surgery were evaluated. Patients were divided into four groups according to incision size (2.2 or 2.8 mm) and with/without stromal hydration; 15 eyes were assigned to each group. The procedures were performed under topical anesthesia by a single surgeon at Inje University Ilsan Paik Hospital. Inclusion criteria were no ocular surgery history, absence of biomicroscopic signs of pseudoexfoliation, normal fundus examination and endothelial cell count of at least 2,000 cells/mm2. Exclusion criteria were the presence of preexisting corneal disease or glaucoma.
Phacoemulsification and intraocular lens implantation were performed as follows: A side-port incision was created and an ophthalmic viscosurgical device was injected into the anterior chamber and then the main clear corneal incision was made using a 2.2- or a 2.8-mm diamond blade in each group. All clear corneal incisions were made temporally. Phacoemulsification was performed using OZil (Alcon, Fort Worth, TX, USA) torsional technology with an ultrasleeve on an Infiniti phacoemulsification platform (Alcon). An intraocular lens (NY-60; HOYA, Tokyo, Japan) was implanted using the cartilage and injector. Corneal stromal hydration was applied in 15 randomly selected eyes in the 2.2- and 2.8-mm incision groups by placing the tip of a 25-gauge cannula in the side walls of the incision and gently irrigating balanced salt solution into the stroma. The remaining 15 eyes in each group were not hydrated.
Incision wounds were measured using AS-OCT (3D-OCT 2000; Topcon, Tokyo, Japan) at 2 hours, 1 day, 1 week, 1 month, and 3 months postoperatively. Anterior module of the machine was used for corneal imaging. During the processes, the patients were asked to look straight ahead to the opposite side of the corneal incisions. Complete transverse scans were taken and the high-definition images were recorded. Corneal thickness at the incision was measured using the anterior OCT module caliper. Incision length and incision angle were also measured and presence of epithelial gaping, endothelial gaping and Descemet's membrane detachment was evaluated.
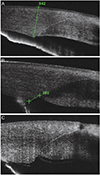
Epithelial gaping was defined as crack of the limbal edge of the superior corneal wound surface, endothelial gaping as crack of the limbal edge of posterior corneal wound surface and Descemet's membrane detachment as separation of Descemet's membrane from the stroma (
Fig. 1).
Statistical analyses of endothelial gaping, corneal thickness and incision length were performed using SPSS ver. 12 (SPSS Inc., Chicago, IL, USA). The independent sample t-tests were used to compare parameters between the two groups (2.2 vs. 2.8 mm, hydration vs. no hydration). Paired t-tests were used to compare the series of postoperative values. A p-value <0.05 was considered to indicate a significant difference. The 95% confidence intervals of the incidence of Descemet's membrane detachment were calculated. The chi-square test was used to compare the incidence values.
Results
In this study 60 eyes of 48 patients were enrolled and each group was composed of 15 eyes that were randomly assigned. The average patient age was 64.26 ± 12.68 years (range, 50 to 77 years). No incidence of postoperative complications such as wound leaking or endophthalmitis was observed in the no stromal hydration group.
2.2-mm incision group
In eyes with stromal hydration, corneal thickness at the clear corneal incision was 1,098.50 ± 67.01, 827.00 ± 85.49, and 722.00 ± 23.20 µm on operation day, 1 week, and 1 month after surgery, respectively. In eyes without stromal hydration, corneal thickness at the clear corneal incision was 1,003.17 ± 22.44, 929.14 ± 67.10, and 797.71 ± 64.01 µm on operation day, 1 week, and 1 month after surgery, respectively.
On operation day, corneal thickness in the 2.2 mm hydration group was statistically significantly thicker than in the 2.2 mm no-hydration group (
p = 0.002). However, at 1 week and 1 month postoperatively, corneal thickness in the 2.2 mm no-hydration group was statistically significantly thicker than in the 2.2 mm hydration group (
p = 0.035,
p = 0.022, respectively) (
Table 1 and
Fig. 1).
Endothelial gaping was statistically significantly larger in the 2.2 mm stromal hydration group than in the 2.2 mm no-hydration group only on operation day (p = 0.041). There was no difference between the two groups postoperatively.
2.8-mm incision group
In eyes with stromal hydration, corneal thickness at the clear corneal incision was 1,138.29 ± 54.18, 872.86 ± 103.93, and 752.86 ± 58.40 µm on operation day, 1 week, and 1 month after surgery, respectively. In eyes without stromal hydration, corneal thickness at the clear corneal incision was 1,021.71 ± 35.95, 889.14 ± 90.20, and 760.14 ± 57.51 µm on operation day, 1 week, and 1 month after surgery, respectively. Corneal thickness was statistically significantly larger in the 2.8 mm stromal hydration group than in the 2.8 mm no-hydration group only on operation day (
p = 0.001). There was no difference between the two groups postoperatively (
Table 2).
Incision size
Corneal thickness was not significantly different between the two groups (2.2 and 2.8 mm) on operation day, 1 day, 1 week, 1 month, and 3 months after surgery.
In eyes with the 2.2-mm incision, endothelial gaping was 169.58 ± 72.72, 80.46 ± 59.73, and 61.00 ± 47.82 at 1 day, 1 month, and 3 months after surgery, respectively. In eyes with the 2.8-mm incision, endothelial gaping was 116.08 ± 41.06, 33.93 ± 23.85, and 0.00 ± 0.00 µm at 1 day, 1 month, and 3 months after surgery, respectively. At 1 day, 1 month, and 3 months postoperatively, endothelial gaping was statistically significantly larger in the 2.2-mm incision group (
p = 0.035,
p = 0.009, and
p = 0.008, respectively). There was no significant difference between the two groups on operation day and 1 week after surgery (
p = 0.131,
p = 0.116) (
Table 3).
Stromal hydration (integrating 2.2- and 2.8-mm incision groups)
In eyes with stromal hydration, corneal thickness at the clear corneal incision was 1,119.92 ± 61.36, 851.69 ± 94.93, and 738.62 ± 46.75 µm on operation day, 1 week, and 1 month after surgery, respectively. In eyes without stromal hydration, corneal thickness at the clear corneal incision was 1013.15 ± 30.80, 909.14 ± 19.14, and 778.93 ± 61.63 µm on operation day, 1 week, and 1 month after surgery, respectively. Only on operation day, corneal thickness was statistically significantly larger in the stromal hydration group than the no-hydration group (p = 0.002). Significant difference in corneal thickness with or without hydration was not observed postoperatively.
Other parameters showed no significant difference between the two groups (with or without stromal hydration) on operation day, 1 day, 1 week, 1 month, and 3 months after surgery (
Table 4).
No stromal hydration
Corneal thickness was only significantly different between the 2 groups (2.2 and 2.8 mm) at 3 months after surgery (770.67 vs. 706.33 µm, p = 0.048).
In eyes with the 2.2-mm incision, endothelial gaping was 247.50 ± 83.30, 204.83 ± 77.12, and 100.00 ± 65.88 µm on operation day, 1 day, and 1 month after surgery, respectively. In eyes with the 2.8-mm incision, endothelial gaping was 157.14 ± 63.60, 114.57 ± 46.80, and 37.01 ± 23.6 7 µm on operation day, 1 day, and 1 month after surgery, respectively. In the 2.8-mm no-hydration group, endothelial gaping was statistically smaller on operation day and 1 day and 1 month postoperatively (
p = 0.035,
p = 0.035, and
p = 0.035, respectively) (
Table 5).
Descemet's membrane detachment
Descemet's membrane detachment was observed in 66.7% (10 / 15 eyes) and 40.0% (6 / 15 eyes) on operation day and 1 day postoperatively, respectively and gradually decreased in the 2.2-mm incision stromal hydration group. In the 2.2-mm no-hydration group, Descemet's membrane detachment was observed in 40% (6 / 15 eyes), 26.7% (4 / 15 eyes), 13.3% (2 / 15 eyes), 0% (0 / 15 eyes), and 0% (0 / 15 eyes) on operation day, 1 day, 1 week, 1 month, and 3 months postoperatively, respectively. In the 2.8-mm incision with stromal hydration group, Descemet's membrane detachment was observed in 40% (6 / 15 eyes), 26.7% (4 / 15 eyes), 26.7% (4 / 15 eyes), 13.3% (2 / 15 eyes), and 0% (0 / 15 eyes) on operation day, 1 day, 1 week, 1 month, and 3 months postoperatively, respectively. In the 2.8-mm no-hydration group, Descemet's membrane detachment was observed in 26.7% (4 / 15 eyes), 26.7% (4 / 15 eyes), 13.3% (2 / 15 eyes), 0% (0 / 15 eyes), and 0% (0 / 15 eyes) on operation day, 1 day, 1 week, 1 month, and 3 months postoperatively, respectively.
In the stromal hydration group (integrating 2.2- and 2.8- mm incision groups), Descemet's membrane detachment was observed in 53.3% (16 / 30 eyes), 33.3% (10 / 30 eyes), 20% (6 / 30 eyes), 13.3% (4 / 15 eyes) and 0% (0 / 15 eyes) on operation day, 1 day, 1 week, 1 month, and 3 months postoperatively, respectively. In the no-hydration group (integrating 2.2- and 2.8-mm incision groups), Descemet's membrane detachment was observed in 33.3% (10 / 30 eyes), 26.7% (8 / 30 eyes), 13.3% (4 / 30 eyes), 0% (0 / 15 eyes) and 0% (0 / 15 eyes) on operation day, 1 day, 1 week, 1 month, and 3 months postoperatively, respectively.
Descemet's detachment gradually decreased in all groups but without statistically significant difference between the two groups including all data from both incision sizes and with or without stromal hydration.
Discussion
AS-OCT has been used to evaluate shape and thickness of cataract incisions, investigate related problems such as epithelial gaping, endothelial gaping and Descemet's membrane detachment and assess early postoperative outcomes [
3,
6,
7,
8,
9,
10]. In our study, AS-OCT was utilized to monitor patients for up to 3 months after surgery.
In the immediate postoperative period, the structural integrity of a clear corneal incision is critical as a barrier against entry of contaminants into the anterior chamber via the tear film. For this reason, incisional stromal hydration is widely used [
6]. Fine et al. [
9] reported that incisional stromal swelling persists for at least 24 hours after surgery, but the duration of stromal swelling after cataract surgery is uncertain. Corneal thickness was shown to increase significantly at 1 week postoperatively in the presence (vs. absence) of stromal hydration, with no significant difference thereafter [
9,
10]. In our patients with 2.2-mm incisions, corneal thickness increased significantly on the day of surgery with the use of hydration. However, at 1 week and 1 month postoperatively, corneal thickness was significantly decreased in hydrated patients (
p = 0.035 and
p = 0.022, respectively). Stromal hydration is characterized by the lateral walls of the main incision with visible whitening of stroma. Although corneal endothelium apparently absolves water content of stromal hydration [
11], a complete understanding of the incisional dynamics in cataract surgery is lacking [
9,
12,
13,
14,
15]. These results imply the process of corneal wound recovery may be intensified and expedited by pumping water onto the corneal endothelium
via stromal hydration. Therefore, we presumed that corneal thickness may remain significantly thinner in the stromal hydration group after surgery.
As previously demonstrated, we similarly detected other early postoperative imperfections of wound architecture, such as endothelial gaping, epithelial gaping and Descemet's membrane detachment. When comparing patients with 2.2- and 2.8-mm incisions (without hydration) on the day of surgery, 1 day, 1 month postoperatively, endothelial gaping was significantly larger when a 2.2-mm incision was used (p = 0.035, p = 0.035, and p = 0.035, respectively). Endothelial gaping is defined by a crack at the limbal edge of the posterior corneal wound surface, which may reflect posterior incisional wound damage. Small incisions may be subject to mechanical damage posteriorly during phacoemulsification, with further damage when stretched by a large lens delivery system. Therefore, we hypothesized that a 2.8-mm incision would cause comparatively less corneal damage in the early postoperative period.
The small incision of MICS may decrease wound leaks, astigmatism and postoperative infection rates, but phacoemulsification of a microincision generally increases the duration and intensity of ultrasound exposure, creates difficulty in handling and exacerbates endothelial cell loss [
1,
2,
3,
4]. Smaller incision size, by virtue of larger ultrasonic pulse duty cycle, lower aspiration flow and lower vacuum is associated with increased wound temperature, which may be damaging [
16]. In fact, we found that endothelial gaping was significantly larger in the early postoperative phase when a small incision was used.
On the day of surgery, we observed Descemet's membrane detachment in 66.7% (10 / 15) of eyes with 2.2-mm incisions and stromal hydration, 40% (6 / 15) of eyes with 2.2-mm incisions and no stromal hydration, 40% (6 / 15) of eyes with 2.8-mm incisions and stromal hydration and 26.7% (4 / 15) of eyes with 2.8-mm incisions with no stromal hydration that gradually disappeared postoperatively in all instances. Descemet's membrane detachment rates reported by Wang et al. [
7] (37.1%, 2.7-mm incision), Dupont- Monod et al. [
10] (51%; 1.3-, 2.2-, and 2.75-mm incisions overall), and Fukuda et al. [
3] (36.7%, 2.4-mm incision) on the day of surgery were comparable. Although none of our data differed significantly based on patient subset, the rates of Descemet's membrane detachment observed with the use of a 2.2-mm incision were higher than previously reported. In our opinion, a 2.2-mm corneal incision may be more vulnerable intraoperatively and during incisional stromal hydration.
Our study had several limitations. First, the patient population was relatively small, only short-term postoperative evaluations were conducted and single OCT scans were obtained for each eye. Further studies are necessary to analyze the long-term structural impact of incision size and stromal hydration in cataract surgery. Second, a difference of corneal thickness according to age or individual existed, but corneal thickness was not measured preoperatively, thus the difference of corneal thickness between the preoperative and postoperative periods could not be compared. Third, vertical length from epithelial to endothelial entry was not measured preoperatively which may have an impact on endothelial gaping.
In conclusion, an AS-OCT system was used to determine the relative vulnerability of a small (2.2-mm) corneal incision during and after cataract surgery. Small incisions may be stretched and damaged by large lens delivery systems or other surgical instrumentation and postoperative stromal hydration may be damaging. Although smaller incisions reflect the latest trend, choosing a surgical technique suitable for the clinical setting is important.
Although a trend can exist, focusing on the trend can cause other inconveniences and problems. The flexibility of using appropriate surgical techniques for patients instead of being preoccupied with either a smaller or larger incision may be more helpful for conducting a safe and efficient cataract surgery.







 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download