Abstract
Purpose
To investigate anterior segment parameters in obese patients in comparison to healthy individuals.
Methods
Thirty-four obese subjects and 34 age-sex-matched healthy subjects were enrolled in this prospective cross-sectional study. Ophthalmological examinations including intraocular pressure (IOP), central corneal thickness (CCT), anterior chamber depth (ACD), anterior chamber volume (ACV), anterior chamber angle (ACA), and axial length (AL) measurements were performed on each subject. Height and weight of all subjects were recorded and body mass index (BMI) was calculated.
Results
IOP was significantly higher in the obese group (p = 0.003). The mean ACD in obese subjects was significantly lower than that in control subjects (p = 0.036). AL, ACV, ACA and CCT were not significantly different between the groups. There was a positive correlation between BMI and IOP (r = 0.404, p < 0.001). ACD and ACA were negatively correlated with BMI.
Obesity is a common health problem in the world. The World Health Organization defines obesity as a body mass index (BMI) of 30 kg/m2 or greater, and overweight as individuals whose BMI falls between 25 kg/m2 and 29.9 kg/m2 [1]. Obesity is an established risk factor for many systemic diseases including hypertension, type 2 diabetes mellitus, coronary heart disease, stroke, dyslipidemia, osteoarthritis and sleep apnea [234].
Obesity has various well-known effects on the cardiovascular and metabolic systems. However the impact of obesity on the eye has not been well documented. Some eye diseases such as glaucoma, cataract, age-related macular degeneration and diabetic retinopathy may be associated with obesity [567891011], though a clear pathophysiological explanation for the association of obesity with eye diseases is currently lacking. Several mechanical and vascular factors or oxidative stress may contribute to the pathogenesis of eye diseases in obese people [8].
The evaluation of several anterior segment parameters such as anterior chamber depth (ACD), anterior chamber volume (ACV), anterior chamber angle (ACA), central corneal thickness (CCT) and axial length (AL) are of paramount importance for ophthalmic examinations. These parameters may provide valuable information for the risk assessment of glaucoma, calculation of intraocular lens power, monitoring of keratoconus and the investigation of refractive disorders [12131415].
Intraocular pressure (IOP) and CCT in obesity have been extensively studied previously however there is no clear data on the effects of obesity on anterior chamber parameters. In the current study, we aimed to evaluate anterior segment parameters and AL in obese people.
The study was performed in adherence to the tenets of the Declaration of Helsinki and the local ethics committee approved the methodology. Written informed consent was obtained from all of the subjects. Thirty-four obese subjects and 34 age-sex-matched healthy subjects (control group) with normal ophthalmological examination were enrolled in this prospective cross-sectional study. Exclusion criteria included smoking habits, history of any ocular disease or previous surgery, presence of systemic diseases such as diabetes mellitus, hypertension, renal or hepatic dysfunction and rheumatological diseases.
BMI was calculated as weight in kilograms divided by the height in meters squared (kg/m2). The participant's height was measured with a wall-mounted tape and weight was measured using a digital scale. Subjects with BMI within the 18.5 to 24.9 range were labeled as normal, while those with BMI higher than 30.0 were labeled as obese [16]. Systolic and diastolic blood pressures were taken with an automated sphygmomanometer.
All subjects underwent a comprehensive ophthalmic examination including medical history review, best-corrected visual acuity, slit-lamp biomicroscopy, tonometry, retinoscopy, biometry and topographic evaluation. IOP was measured with a Goldmann applanation tonometer (Haag-Streit, Koeniz, Switzerland). The anterior segment parameters were evaluated using the Scheimpflug camera with a Placido disc topographer (Sirius; Costruzione Strumenti Oftalmici, Florence, Italy). Measurements were conducted with undilated pupils under scotopic conditions by a single experienced technician. Scans were taken in automatic mode with proper quality. The following parameters were extracted from the obtained topographic and pachymetric maps for statistical analysis; CCT, ACD, ACV, and ACA. Furthermore, AL was measured with ocular biometry (LenStar LS900, Haag-Streit).
Data analysis was performed using SPSS ver. 11.5 (SPSS Inc., Chicago, IL, USA). The Kolmogorov Smirnov test was used to determine whether the distributions of metric discrete and continuous variables were normal. Metric discrete and continuous variables were shown as mean ± standard deviation or median, where applicable. The paired t-test was used to evaluate whether the mean differences in clinical measurements between the right and left side were statistically significant; otherwise, the Wilcoxon signed rank test was applied for comparisons of the median values. While the mean differences between groups were compared using Student's t-test, the Mann-Whitney U-test was used for comparisons of the median values. The relationship between clinical measurements was analyzed by Spearman's rank correlation test. A p-value of less than 0.05 was considered statistically significant.
The mean age of the obese and control group was 49.3 ± 7.6 and 48.5 ± 9.4 years, respectively. Data on the age, sex and BMI of the subjects are presented in Table 1. There was a significant difference between the groups in terms of BMI (p < 0.01). As there were no significant differences between the measurements of right and left eyes, only the readings of the left eyes were used for analysis (Table 2).
The mean IOP was 15 mmHg (range, 10 to 22 mmHg) in the obese group and 13 mmHg (range, 8 to 20 mmHg) in the control group. IOP was significantly higher in the obese group (p = 0.003) (Table 3). The mean ACD in obese subjects was significantly lower than in control subjects (p = 0.036). AL, ACV, ACA, and CCT were not significantly different between the two groups. There was a positive correlation between BMI and IOP (r = 0.404, p < 0.001). ACD and ACA were negatively correlated with BMI (Table 4).
Results of correlation analysis for anterior chamber parameters revealed a strong positive correlation between ACD and ACA (r = 0.763, p < 0.001) (Fig. 1). A moderate but significant correlation was found between ACD and ACV (r = 0.647, p < 0.001) (Fig. 2), as well as between ACV and ACA (r = 0.531, p < 0.001) (Fig. 3).
To the best of our knowledge, this is the first study to evaluate anterior segment parameters in obese patients. This study demonstrated that IOP was significantly higher and ACD was significantly lower in obese patients. In addition, AL, ACV, ACA, and CCT values were similar in obese and healthy subjects.
Obesity occurs when energy intake exceeds energy consumption. In accordance with the definition of obesity, health risk is related to fat in relation to total body mass; so it is necessary to determine the total mass of fat and the ratio of fat to total body weight [17]. Fat storage varies based on obesity such that white adipose tissue can be found in many areas of the body, including the face, extremities, abdomen, omentum, intestines, thighs, bone marrow and retro-bulbar space [1819]. Fat deposition in the orbita leads to orbital volume enlargement. Increased orbital pressure by mechanism of "mass effect" may cause a decrease in episcleral aqueous outflow, inducing elevated IOP [1920]. This mechanism can be demonstrated by orbital space lesions, such as Graves' ophthalmopathy and carotid-cavernous fistula. In support of this theory, retrobulbar injection of anesthetics may lead to increased IOP [21]. The influence of BMI upon IOP has been reported in several studies. Stojanov et al. [19] showed obese subjects had significantly higher IOP than subjects with normal weight (15.96 vs. 12.99 mmHg, p < 0.01). Mori et al. [22] found elevated IOP in subjects with BMI over 25 in Japan. Zafra Perez et al. [23] suggested that elevated IOP is associated with obesity. In this study, we found IOP was significantly higher in the obese group compared to controls (15 vs. 13 mmHg, p = 0.003). Additionally, IOP was positively correlated with BMI (r = 0.404, p < 0.001), similar to the results of Jang et al. [24].
Elevated IOP in obese people may be facilitated by several mechanisms. One hypothesis asserts that increased orbital fat and blood viscosity increases the episcleral venous pressure and reduces the outflow of aqueous, which results in increased IOP [1920]. The other theory is that obesity linked hyperleptinemia may cause oxidative damage to the trabecular meshwork [25].
Since increased IOP is the major risk factor for the development of glaucomatous optic neuropathy, it is important to assess how the structures of the anterior chamber and CCT affect IOP. Shallow ACD was reported as a strong risk factor for angle closure glaucoma [2627]. In the obese group, we observed a significantly shallower anterior chamber (decrease in ACD) and narrower ACA as BMI increased. Increased retrobulbar fat volume also plays a critical role in elevated IOP in addition to other factors linked to IOP elevation in obesity such as increased episcleral venous pressure, increased blood viscosity and aqueous humor outflow disorders [28]. Retrobulbar fat is clearly limited by the orbital space. Due to this limitation there is no possibility of expansion, unlike other fat tissue depots in the body [19]. This limitation may cause a decrease in ACD and ACA depending on BMI and fat deposits. Although the relationship of obesity and biometry measurements such as ACD and ACA has not been well studied, obese individuals were noted to have more hyperopic vision and shorter vitreous chambers [2930]. Moreover, obesity has been reported as a risk factor for cataracts [56]. Although a significant decrease in ACD was found with an increase in lens thickness [31], the complex relationship between obesity, cataract type and lens thickness is still controversial [8].
CCT is an important factor in the clinical evaluation of several eye disorders, especially glaucoma. In the current study, differences in CCT values between the groups were not statistically significant. Similarly, in a previous study, Turkish researchers did not find a significant difference in CCT of normal subjects and patients with metabolic syndrome [32]. Nonetheless, in contrast with our results, several studies have reported higher CCT readings with increasing BMI [3334]. In the literature, there is a strong relationship between BMI and IOP, but the relationship between high BMI and glaucomatous optic neuropathy remains unclear [25]. Su et al. [33] explained this inconsistency with the impact of CCT on IOP measurements. People with high BMI have thicker corneas resulting in higher IOP readings. However, individuals with high CCT are less likely to develop glaucoma. We are unable to explain why CCT readings between our groups were not significant, though it may be related with sample size and nature. Still, we can conclude that we found a significant association between IOP and obesity, independent of the effect of CCT.
AL is the distance from the corneal surface to an interference peak corresponding to the retinal pigment epithelium [33]. In adults, AL remains practically unchanged but myopes tend to have a longer AL and hypermetropes tend to have shorter AL as compared to those with emmetropes [34]. Although AL was significantly associated with the higher body height [35], there is no clear data about the effect of obesity on the measurements of the AL. Previous studies have demonstrated that short AL is a strong risk factor for angle closure glaucoma [2627]. In our study, we could not able to find a significant difference in AL between groups.
There are several limitations of the current study. It is a single-center study and has a relatively small sample size. Therefore, these findings need to be confirmed in a larger patient group.
In conclusion, IOP was significantly higher and ACD was significantly lower in obese subjects. AL, ACV, ACA and CCT were not significantly different between the groups. The impact of obesity on anterior chamber parameters should be further investigated.
Figures and Tables
 | Fig. 1Scatter plot between anterior chamber angle (ACA) and anterior chamber depth (ACD) measurements. |
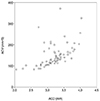 | Fig. 2Scatter plot between anterior chamber volume (ACV) and anterior chamber depth (ACD) measurements. |
 | Fig. 3Scatter plot between anterior chamber angle (ACA) and anterior chamber volume (ACV) measurements. |
References
1. World Health Organization. Controlling the global obesity epidemic. Geneva: World Health Organization;2002. p. 60.
2. Eckel RH, Krauss RM. AHA Nutrition Committee. American Heart Association call to action: obesity as a major risk factor for coronary heart disease. Circulation. 1998; 97:2099–2100.
3. Grundy SM. Metabolic complications of obesity. Endocrine. 2000; 13:155–165.
4. Lawrence VJ, Kopelman PG. Medical consequences of obesity. Clin Dermatol. 2004; 22:296–302.
5. Leske MC, Wu SY, Hennis A, et al. Diabetes, hypertension, and central obesity as cataract risk factors in a black population: the Barbados Eye Study. Ophthalmology. 1999; 106:35–41.
6. Kuang TM, Tsai SY, Hsu WM, et al. Body mass index and age-related cataract: the Shihpai Eye Study. Arch Ophthalmol. 2005; 123:1109–1114.
7. Leske MC, Connell AM, Wu SY, et al. Risk factors for open-angle glaucoma: the Barbados Eye Study. Arch Ophthalmol. 1995; 113:918–924.
8. Cheung N, Wong TY. Obesity and eye diseases. Surv Ophthalmol. 2007; 52:180–195.
9. Van Leiden HA, Dekker JM, Moll AC, et al. Blood pressure, lipids, and obesity are associated with retinopathy: the hoorn study. Diabetes Care. 2002; 25:1320–1325.
10. Seddon JM, Cote J, Rosner B. Progression of age-related macular degeneration: association with dietary fat, transunsaturated fat, nuts, and fish intake. Arch Ophthalmol. 2003; 121:1728–1737.
11. Maralani HG, Tai BC, Wong TY, et al. Metabolic syndrome and risk of age-related macular degeneration. Retina. 2015; 35:459–466.
12. Nemeth J, Fekete O, Pesztenlehrer N. Optical and ultrasound measurement of axial length and anterior chamber depth for intraocular lens power calculation. J Cataract Refract Surg. 2003; 29:85–88.
13. Abolbashari F, Mohidin N, Ahmadi Hosseini SM, et al. Anterior segment characteristics of keratoconus eyes in a sample of Asian population. Cont Lens Anterior Eye. 2013; 36:191–195.
14. Lee Y, Sung KR, Na JH, Sun JH. Dynamic changes in anterior segment (AS) parameters in eyes with primary angle closure (PAC) and PAC glaucoma and open-angle eyes assessed using AS optical coherence tomography. Invest Ophthalmol Vis Sci. 2012; 53:693–697.
15. Malyugin BE, Shpak AA, Pokrovskiy DF. Accommodative changes in anterior chamber depth in patients with high myopia. J Cataract Refract Surg. 2012; 38:1403–1407.
16. Tzamaloukas AH, Murata GH, Hoffman RM, et al. Classification of the degree of obesity by body mass index or by deviation from ideal weight. JPEN J Parenter Enteral Nutr. 2003; 27:340–348.
17. Wells JC, Victora CG. Indices of whole-body and central adiposity for evaluating the metabolic load of obesity. Int J Obes (Lond). 2005; 29:483–489.
18. Gesta S, Tseng YH, Kahn CR. Developmental origin of fat: tracking obesity to its source. Cell. 2007; 131:242–256.
19. Stojanov O, Stokic E, Sveljo O, Naumovic N. The influence of retrobulbar adipose tissue volume upon intraocular pressure in obesity. Vojnosanit Pregl. 2013; 70:469–476.
20. Otto AJ, Koornneef L, Mourits MP, et al. Retrobulbar pressures measured during surgical decompression of the orbit. Br J Ophthalmol. 1996; 80:1042–1045.
21. Nassr MA, Morris CL, Netland PA, Karcioglu ZA. Intraocular pressure change in orbital disease. Surv Ophthalmol. 2009; 54:519–544.
22. Mori K, Ando F, Nomura H, et al. Relationship between intraocular pressure and obesity in Japan. Int J Epidemiol. 2000; 29:661–666.
23. Zafra Perez JJ, Villegas Perez MP, Canteras Jordana M, Miralles De Imperial J. Intraocular pressure and prevalence of occult glaucoma in a village of Murcia. Arch Soc Esp Oftalmol. 2000; 75:171–178.
24. Jang HD, Kim DH, Han K, et al. Relationship between intraocular pressure and parameters of obesity in Korean adults: the 2008-2010 Korea National Health and Nutrition Examination Survey. Curr Eye Res. 2014; 11. 07. DOI: 10.3109/02713683.2014.975367.
25. Newman-Casey PA, Talwar N, Nan B, et al. The relationship between components of metabolic syndrome and open-angle glaucoma. Ophthalmology. 2011; 118:1318–1326.
26. Lavanya R, Wong TY, Friedman DS, et al. Determinants of angle closure in older Singaporeans. Arch Ophthalmol. 2008; 126:686–691.
27. Lowe RF. Aetiology of the anatomical basis for primary angle-closure glaucoma. Biometrical comparisons between normal eyes and eyes with primary angle-closure glaucoma. Br J Ophthalmol. 1970; 54:161–169.
28. Halpern DL, Grosskreutz CL. Glaucomatous optic neuropathy: mechanisms of disease. Ophthalmol Clin North Am. 2002; 15:61–68.
29. Saw SM, Chua WH, Hong CY, et al. Height and its relationship to refraction and biometry parameters in Singapore Chinese children. Invest Ophthalmol Vis Sci. 2002; 43:1408–1413.
30. Wong TY, Foster PJ, Johnson GJ, et al. The relationship between ocular dimensions and refraction with adult stature: the Tanjong Pagar Survey. Invest Ophthalmol Vis Sci. 2001; 42:1237–1242.
31. Praveen MR, Vasavada AR, Shah SK, et al. Lens thickness of Indian eyes: impact of isolated lens opacity, age, axial length, and inf luence on anterior chamber depth. Eye (Lond). 2009; 23:1542–1548.
32. Sahinoglu-Keskek N, Keskek SO, Cevher S, et al. Metabolic syndrome as a risk factor for elevated intraocular pressure. Pak J Med Sci. 2014; 30:477–482.
33. Su DH, Wong TY, Foster PJ, et al. Central corneal thickness and its associations with ocular and systemic factors: the Singapore Malay Eye Study. Am J Ophthalmol. 2009; 147:709–716.e1.
34. Nishitsuka K, Kawasaki R, Kanno M, et al. Determinants and risk factors for central corneal thickness in Japanese persons: the Funagata Study. Ophthalmic Epidemiol. 2011; 18:244–249.
35. Yin G, Wang YX, Zheng ZY, et al. Ocular axial length and its associations in Chinese: the Beijing Eye Study. PLoS One. 2012; 7:e43172.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download