Abstract
Purpose
To analyze differences in the subfoveal choroidal thickness (SFChT) between bevacizumab responders (BevRs) and nonresponders (BevNRs) in patients with idiopathic central serous chorioretinopathy (CSC).
Methods
The medical records of 30 unilateral chronic CSC patients who were treated with intravitreal bevacizumab (IVB) as a first line treatment were reviewed. Patients were categorized as BevNRs when CSC did not completely resolve after a minimum of 3 IVB treatments. Enhanced depth imaging-optical coherence tomography was used and SFChT was measured before and after treatment. Choroidal hyperpermeability was also evaluated using indocyanine angiography.
Results
Twenty and 10 eyes were classified as BevRs or BevNRs, respectively. The mean number of IVB treatments was 2.22 ± 0.89 in BevRs, and 4.80 ± 1.03 in BevNRs. Compared with BevNRs, BevRs demonstrated significantly greater pretreatment SFChT (441.25 ± 88.09 vs. 364.10 ± 61.97 µm); SFChT reduction following IVB was significantly greater in BevRs than BevNRs. SFChT in the unaffected eyes was also greater in BevRs than BevNRs. Choroidal hyperpermeability was detected less frequently in BevNRs (hypofluorescence on late-phase, 0.0% and 33.3% in BevNRs and BevRs, respectively; p= 0.049).
Conclusions
Compared with CSC eyes that did not respond well to IVB, BevRs demonstrated significantly thicker SFChT at baseline, greater reduction in SFChT after IVB treatment, and hyperfluorescence on late-phase indocyanine green angiography. We recommend IVB injection as the first-line therapy for CSC eyes with relatively high SFChT and hyperfluorescence on late-phase indocyanine green angiography.
Central serous chorioretinopathy (CSC) is characterized by well-demarcated serous neurosensory retinal detachment in the posterior pole. CSC is a self-limiting disease
that is usually associated with a good visual prognosis. In some cases, however, persistent CSC results in permanent damage to the retina or retinal pigment epithelium [1,2,3,4,5]. Therefore, if the disease continues beyond the acute phase, active treatment must be considered to prevent irreversible damage to retinal function.
The pathogenesis of CSC is associated with abnormal choroidal circulation and/or abnormal barrier function in the retinal pigment epithelium. Indocyanine green angiography (ICGA) shows dilated choroidal vessels and choroidal hyperpermeability in CSC patients [6]. The recent development of enhanced depth image-optical coherence tomography (EDI-OCT) has improved the accuracy of choroidal examinations with better quantitative results and increased reliability [7,8,9]. Several EDI-OCT investigations have analyzed the choroidal thickness of CSC eyes. The results of these investigations reveal greater choroidal thickness in CSC eyes relative to age-matched normal eyes. Furthermore, choroidal thickness in unaffected contralateral eyes is also greater than in age-matched controls [10,11,12,13,14].
Intravitreal bevacizumab (IVB) injection is a therapeutic option for chronic CSC. Studies that evaluated the efficacy of IVB injection in CSC eyes have found good visual and anatomical outcomes [15,16,17,18,19,20]. However, IVB administration is not effective in all cases of chronic CSC, which necessitates consideration of alternative treatments such as focal argon laser photocoagulation or photodynamic therapy with verteporfin.
Inoue et al. [21] demonstrated that photodynamic therapy for chronic CSC was not effective in eyes without intense hyperfluorescence on late-phase ICGA. Moreover, Jirarattanasopa et al. [22] documented a connection between choroidal thickness and choroidal vascular hyperpermeability on ICGA. They reported that choroidal thickness is greater in areas with choroidal vascular hyperpermeability on ICGA than in unaffected areas [21,22]. Based on these observations, we hypothesized that the responsiveness of specific CSC patients to IVB injection might be related to late-phase ICGA findings and pretreatment choroidal thickness. Given the absence of prior studies on this issue, we investigated differences in pretreatment and posttreatment subfoveal choroidal thickness (SFChT) and late-phase ICGA findings between bevacizumab responders (BevRs) and nonresponders (BevNRs) using Heidelberg Spectralis EDI-OCT and ICGA.
A retrospective review was conducted of all patients who were diagnosed with CSC at Asan Medical Center in Seoul, Korea, from October 2010 through October 2012. The following inclusion criteria were used: (1) definite presence of subretinal fluid on spectral domain (SD) OCT, (2) visual disturbance persisting ≥3 months, (3) evidence of diffuse and/or focal leaking on fluorescein angiography (FA), (4) the use of IVB injection as a first-line treatment option for idiopathic CSC, and (5) ≥6-month follow-up after the first intravitreal bevacizumab injection. Patients were excluded based on the presence of refractive errors <±4.0 diopters, presence of bilateral CSC, choroidal neovascularization or other macular diseases that might affect vision, active intraocular inflammation and/or infection, or a history of any type of intraocular surgery (including cataract surgery). Patients with CSC caused by systemic steroid use were also excluded. The study was approved by the institutional review board of Asan Medical Center and followed the tenets of the Declaration of Helsinki.
BevRs were defined as bevacizumab-responsive patients that had complete resolution of subretinal fluid on SD-OCT after ≤3 IVB treatments. BevNRs were defined as bevacizumab-nonresponsive patients if subretinal fluid did not completely resolve after a minimum of three IVB injections. Bevacizumab (1.25 mg, 0.05 mL) was injected in CSC eyes at 6-week intervals. Information about symptom duration, medical/medication history, and the total number of bevacizumab injections was obtained from the medical records of each study participant.
The primary objective of this study was to analyze differences in pretreatment SFChT between BevR and BevNR patients. The secondary objectives of this study were to (1) determine changes in SFChT after administering IVB to each group, (2) determine differences in SFChT in the contralateral eye between BevR and BevNR patients, (3) determine the relationship between ICGA findings and bevacizumab responsiveness, and (4) determine the baseline characteristics of each group.
All patients received a complete bilateral ophthalmic examination, including pretreatment and posttreatment testing of best-corrected visual acuity (BCVA) using the Snellen eye chart. All BCVA results were converted to the logarithm of the minimum angle of resolution (logMAR) scale. All patients also underwent refractive error assessment, biomicroscopic examination, fundus examination, fundus photography, FA, and SD-OCT. ICGA was performed, as needed, to rule out choroidal neovascularization. Heidelberg Retina Angiograph 2 (Heidelberg Engineering, Heidelberg, Germany) with a 30° field of view was used to assess FA and ICGA images. Heidelberg Spectralis (Heidelberg Engineering) was used to obtain SD-OCT images of the macula using a custom 25° × 25° volume acquisition protocol to obtain a set of high-speed scans from each eye. Using this protocol, 25 cross-sectional B-scan images were obtained, each of which was composed of 512 A-scans. Choroidal images were also obtained using the EDI technique. After intravitreal injection of bevacizumab, SD-OCT was performed on the affected eye at each visit to the clinic.
SFChT was manually measured by two retina specialists (DYK and SGJ) using calipers and Heidelberg Eye Explorer software. For BevR, SFChT was measured 3 months after anatomical resolution of subretinal fluid. For BevNR, SFChT was measured at the most recent follow-up examination or just before changes in treatment. SFChT was vertically measured from the outer border of the retinal pigment epithelium to the inner border of the sclera (Fig. 1). After the first operator (DYK) assessed the SFChT, the second operator (SGJ) assessed the same parameter according to the same protocol without knowledge of the first operator's results. The mean data of two different measurements were used in this study. Normal control data were obtained from 55 contra-lateral eyes of age-matched patients who had unilateral disease, such as idiopathic epiretinal membrane, macular hole, ocular trauma, or rhegmatogenous retinal detachment.
Late-phase ICGA data obtained 10 to 15 minutes after dye injection was used to classify each eye according to the presence of hyperfluorescence or hypofluorescence. Fig. 2 shows the late-phase ICGA findings of CSC eyes. Hyperfluorescence was defined as the presence of well-defined homogeneous spots with a higher than background fluorescence, whereas hypofluorescence was defined as no distinct fluorescence or slightly decreased fluorescence compared with background fluorescence [22,23].
The Mann-Whitney test was used to compare pretreatment SFChT, posttreatment SFChT, and SFChT in the contralateral eyes between the BevR and BevNR groups. The Fisher's exact test was used to analyze the relationship between each group (responsive or nonresponsive) and ICGA findings. Clinical characteristics were compared between the BevNR and BevR groups by applying the Mann-Whitney test. IBM SPSS ver. 21.0 (IBM Corp., Armonk, NY, USA) was used to perform all analyses, and p< 0.05 was considered statistically significant.
Thirty patients (n = 30 affected eyes) satisfied the inclusion criteria for the current study among patients who were diagnosed with CSC at the Asan Medical Center between October 2010 through October 2012.
Among the 30 included patients, 20 patients/eyes were further classified as BevRs and assigned to the BevR group; 10 patients/eyes were classified as BevNRs and assigned to the BevNR group. Table 1 shows the mean pre-and posttreatment SFChT values. The difference between the mean pretreatment and posttreatment SFChT values (delta SFChT) is also shown for each group. The mean pretreatment SFChT value was significantly greater in the BevR than BevNR group. Furthermore, the extent of subfoveal choroidal thinning after IVB injection was significantly greater in the BevR than in the BevNR group (delta SFChT, 63.25 ± 44.53 vs. -3.10 ± 37.71 µm, p< 0.001).
Fig. 3 shows the pretreatment and posttreatment SFChT values, and SFChT values in the unaffected contralateral eye, and SFChT in the healthy controls. The mean SFChT of the BevR group was significantly reduced from 441.25 ± 88.09 to 378.00 ± 79.04 µm after IVB injection (p< 0.001). However, the mean SFChT value of the BevNR group was not significantly altered (pretreatment, 364.10 ± 61.97 µm; posttreatment, 367.20 ± 53.45 µm; p= 0.838). Moreover, the pretreatment SFChT values of the unaffected contralateral eyes in the BevNR group were significantly smaller compared with the unaffected contralateral eyes in the BevR group (BevNR, 313.50 ± 62.94 µm; BevR, 403.72 ± 90.96 µm; p= 0.019). Compared with SFChT of the healthy control (281.93 ± 53.91 µm), SFChT of the unaffected contralateral eye was significantly higher in the BevRs (403.39 ± 90.96 µm, p< 0.001), but not in the BevNRs (319.75 ± 65.98 µm, p= 0.069).
The relationship between bevacizumab responsiveness and late-phase ICGA is illustrated in Table 2. The BevNR group demonstrated a statistically significant correlation between late-phase ICGA hypofluorescence and a lack of IVB injection responsiveness (Fisher's exact test, 33.3% in BevNR vs. 0.0% BevR; p= 0.049).
Table 3 shows the baseline characteristics of BevR and BevNR. The symptom duration was not significantly different between BevR and BevNR (BevNR, 8.30 ± 5.3 months; BevR, 7.00 ± 4.14 months; p= 0.114). Age, refractive error, and follow-up duration were not significantly different between groups. Pretreatment BCVA was significantly lower in the BevNR group relative to the BevR group. Fig. 4 shows the correlation between symptom duration and pretreatment logMAR BCVA. There was no significant correlation between symptom duration and pretreatment logMAR BCVA according to the Pearson correlation analysis (p= 0.474). The total injection number was significantly greater in the BevNR than in the BevR group (BevNR, 4.80 ± 1.03; BevR, 2.22 ± 0.89; p< 0.001). In the BevR group, 20 patients (100.0%) achieved complete resolution of subretinal fluid following ≤3 IVB injections. In the BevNR group, the subretinal fluid was unchanged in 9 eyes, and increased in 1 eye, after three IVB injections.
While IVB injection is an important therapeutic option for managing chronic CSC, not all cases of chronic CSC patients respond to IVB administration. In the current study, EDI-OCT and ICGA analysis determined that (1) the thickening of the subfoveal choroidal layer was less prominent in BevNR-CSC eyes in comparison with BevR-CSC eyes at baseline, (2) SFChT reduction after IVB injection was significantly greater in BevRs than BevNRs, (3) BevNR eyes demonstrated a higher rate of hypof luorescence on late-phase ICGA, and (4) SFChT in the unaffected contralateral eye was higher in comparison with healthy controls, but was significantly thicker only in BevRs.
SFChT thickening in CSC eyes has already been reported, but there are a limited number of studies on treatment response according to the amount of SFChT thickening in CSC eyes. The current study found that compared with CSC eyes that responded well to IVB, non-responding eyes had significantly thinner SFChT at baseline. The study also found that among CSC eyes with increased SFChT, CSC eyes with relatively smaller increases in SFChT demonstrated poor IVB response. Considering the relatively thin SFChT of the unaffected contralateral eyes in the BevNR group, this suggests that BevRs and BevNRs might demonstrate different pathogenic mechanisms and characteristics.
Classically, barrier function defect in the retinal pigment epithelium is considered to be the main pathogenesis mechanism in CSC eyes. However, advances in ICGA indicate that abnormal choroidal vasculature might be the primary pathogenic cause of CSC [1,6]. If the abnormal choroidal vasculature is the main pathogenic mechanism of CSC eyes, the role of VEGF might be emphasized and provide legitimacy of the anti-VEGF therapy to CSC eyes. The present study found that responding eyes exhibited significantly thicker SFChT at baseline and a higher rate of hyperfluorescence on late-phase ICGA. These results are consistent with Jirarattanasopa et al. [22] on the association between choroidal thickness and choroidal vascular hyperpermeability on ICGA. Furthermore, Inoue et al. [21] reported that the eyes of chronic CSC patients without intense hyperfluorescence demonstrate inadequate response to photodynamic therapy. Although the treatment modalities differ between studies, the major finding that treatment nonresponders demonstrate hypof luorescence on late-phase ICGA was common to both studies.
This study analyzed the total number of IVB injections per patient. The total number of injections was significantly greater in BevNR than BevR patients (BevR, 2.22 ± 0.89; BevNR, 4.80 ± 1.03; p< 0.001). Because intravitreal injections inevitably lead to serious complications, such as endophthalmitis and retinal detachment, clinicians have sought guidelines that reduce and/or prevent ineffective drug administration [24,25]. Our findings demonstrate that the basic characteristics of BevNR patients include: decreased SFChT, hypofluorescence on late-phase ICGA, and low pretreatment BCVA. Therefore, by considering these characteristics in BevNRs, we could predict whether individual CSC eyes will respond to IVB treatment.
Choroidal thickness is potentially affected by age, axial length, refractive error, and diurnal variation [26,27,28,29,30,31]. In our current analysis, age was not significantly different between the study groups. Because of the retrospective design of our present study, axial length was not available for each patient. However, refractive error was not significantly different between the BevR and BevNR groups. Diurnal variation in choroidal thickness was reported previously by Usui et al. [27] and Tan et al. [28]. These authors reported that choroidal thickness is greatest in the morning and progressively decreases throughout the day [27,28]. In our current analyses, EDI-OCT images were obtained at an undesignated time; therefore, diurnal variation could not be taken into account. However, according to previous studies, the diurnal variation of choroidal thickness is 20 to 30 µm [27,28]. These relatively small amounts of diurnal variation would not have changed the difference in SFChT between BevNRs and BevRs.
Our study has limitations that are inherent to its retrospective design. The sample sizes of the BevR and BevNR groups were relatively small. This may limit the statistical power in distinguishing the differences between BevRs and BevNRs. Furthermore, we did not include an untreated control group of CSC eyes. It is possible that some BevR patients experienced spontaneous improvement or resolution that coincided with IVB treatment. However, we minimized this possibility by excluding patients with symptom duration that was <3 months. Future studies that examine a larger number of patients and include an untreated control group may be able to define the baseline characteristics of BevNRs.
In conclusion, the basic clinical characteristics of chronic CSC patients who are unresponsive to IVB treatment include low pretreatment BCVA, relatively thin SFChT, and hypofluorescence on late-phase ICGA. Based on the results of this study, we recommend IVB injection as the first-line therapy for chronic CSC eyes with relatively thick SFChT and hyperfluorescence on late-phase ICGA, but not chronic CSC eyes with reduced SFChT and hypofluorescence on late-phase ICGA.
Figures and Tables
 | Fig. 1Subfoveal choroidal thickness measurements. (a) Subfoveal choroidal thickness was vertically measured from the outer border of the retinal pigment epithelium to the inner border of the sclera. |
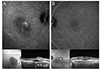 | Fig. 2Late-phase indocyanine green angiography findings and the subfoveal choroidal thickness (SFChT) in central serous chorioretinopathic eyes. Late-phase indocyanine green angiography findings obtained between 10 to 15 minutes after dye injection were used to classify each eye as hyperfluorescent or hypofluorescent. (A) Late-phase hyperfluorescence was defined as the presence of well-defined homogeneous spots that were brighter than background fluorescence. Central serous chorioretinopathy eyes with late-phase hyperfluorescence and relatively thick SFChT are shown. (B) Late-phase hypofluorescence was defined as the presence of an area with no distinct fluorescence or slightly decreased fluorescence in comparison with background fluorescence. Central serous chorioretinopathy eyes with late-phase hypofluorescence and relatively thin SFChT are shown. |
 | Fig. 3Comparison of subfoveal choroidal thickness (SFChT). SFChT in bevacizumab responders (BevRs) was significantly greater than in bevacizumab nonresponders (BevNRs) (*Mann-Whitney test, p < 0.001). Significant differences were determined between the pretreatment and posttreatment SFCT values in BevRs (†Wilcoxon signed-rank test, p < 0.001). The SFChT values of the unaffected contralateral eye were also significantly different between BevRs and BevNRs (‡Mann-Whitney test, p = 0.026). The SFChT values of the unaffected contralateral eye were significantly greater in BevRs than in healthy controls (§Mann-Whitney test, p < 0.001). |
 | Fig. 4Correlation between symptom duration and pretreatment logarithm of the minimum angle of resolution (logMAR) best-corrected visual acuity (BCVA). There was no significant correlation between symptom duration and pretreatment logMAR BCVA (Pearson correlation analysis, p = 0.474) |
Acknowledgements
This study was supported by a grant from the Ministry of Science, ICT and Future Planning, Republic of Korea (NRF-2013R1A2A2A10168457).
References
1. Dohrmann J, Lommatzsch A, Spital G, Pauleikhoff D. Pathogenesis of central serous chorioretinopathy: angiographic and electrophysiological studies. Ophthalmologe. 2001; 98:1069–1073.
2. Piccolino FC, de la Longrais RR, Ravera G, et al. The foveal photoreceptor layer and visual acuity loss in central serous chorioretinopathy. Am J Ophthalmol. 2005; 139:87–99.
3. Wong R, Chopdar A, Brown M. Five to 15 year follow-up of resolved idiopathic central serous chorioretinopathy. Eye. 2004; 18:262–268.
4. Loo RH, Scott IU, Flynn HW Jr, et al. Factors associated with reduced visual acuity during long-term follow-up of patients with idiopathic central serous chorioretinopathy. Retina. 2002; 22:19–24.
5. Iida T, Yannuzzi LA, Spaide RF, et al. Cystoid macular degeneration in chronic central serous chorioretinopathy. Retina. 2003; 23:1–7.
6. Yannuzzi LA, Slakter JS, Gross NE, et al. Indocyanine green angiography-guided photodynamic therapy for treatment of chronic central serous chorioretinopathy: a pilot study. Retina. 2003; 23:288–298.
7. Wong IY, Koizumi H, Lai WW. Enhanced depth imaging optical coherence tomography. Ophthalmic Surg Lasers Imaging. 2011; 42:S75–S84.
8. Yamashita T, Yamashita T, Shirasawa M, et al. Repeatability and reproducibility of subfoveal choroidal thickness in normal eyes of Japanese using different SD-OCT devices. Invest Ophthalmol Vis Sci. 2012; 53:1102–1107.
9. Branchini L, Regatieri CV, Flores-Moreno I, et al. Reproducibility of choroidal thickness measurements across three spectral domain optical coherence tomography systems. Ophthalmology. 2012; 119:119–123.
10. Kuroda S, Ikuno Y, Yasuno Y, et al. Choroidal thickness in central serous chorioretinopathy. Retina. 2013; 33:302–308.
11. Yang SJ, Chen CY, Chang GD, et al. Activation of Akt by advanced glycation end products (AGEs): involvement of IGF-1 receptor and caveolin-1. PLoS One. 2013; 8:e58100.
12. Margolis R, Spaide RF. A pilot study of enhanced depth imaging optical coherence tomography of the choroid in normal eyes. Am J Ophthalmol. 2009; 147:811–815.
13. Maruko I, Iida T, Sugano Y, et al. Subfoveal choroidal thickness in fellow eyes of patients with central serous chorioretinopathy. Retina. 2011; 31:1603–1608.
14. Kim YT, Kang SW, Bai KH. Choroidal thickness in both eyes of patients with unilaterally active central serous chorioretinopathy. Eye (Lond). 2011; 25:1635–1640.
15. Lim JW, Kim MU. The efficacy of intravitreal bevacizumab for idiopathic central serous chorioretinopathy. Graefes Arch Clin Exp Ophthalmol. 2011; 249:969–974.
16. Huang WC, Chen WL, Tsai YY, et al. Intravitreal bevacizumab for treatment of chronic central serous chorioretinopathy. Eye (Lond). 2009; 23:488–489.
17. Schaal KB, Hoeh AE, Scheuerle A, et al. Intravitreal bevacizumab for treatment of chronic central serous chorioretinopathy. Eur J Ophthalmol. 2009; 19:613–617.
18. Lim SJ, Roh MI, Kwon OW. Intravitreal bevacizumab injection for central serous chorioretinopathy. Retina. 2010; 30:100–106.
19. Semeraro F, Romano MR, Danzi P, et al. Intravitreal bevacizumab versus low-f luence photodynamic therapy for treatment of chronic central serous chorioretinopathy. Jpn J Ophthalmol. 2012; 56:608–612.
20. Lee YS, Ha JK, Kim YJ, et al. Comparative outcome analysis of malpositioned and properly positioned fixation groups after hamstring autograft ACL reconstruction with femoral cross-pin fixation. Knee. 2011; 18:30–33.
21. Inoue R, Sawa M, Tsujikawa M, Gomi F. Association between the efficacy of photodynamic therapy and indocyanine green angiography findings for central serous chorioretinopathy. Am J Ophthalmol. 2010; 149:441–446.
22. Jirarattanasopa P, Ooto S, Tsujikawa A, et al. Assessment of macular choroidal thickness by optical coherence tomography and angiographic changes in central serous chorioretinopathy. Ophthalmology. 2012; 119:1666–1678.
23. Lim SH, Chang W, Sagong M. Efficacy of half-fluence photodynamic therapy depending on the degree of choroidal hyperpermeability in chronic central serous chorioretinopathy. Eye (Lond). 2013; 27:353–362.
24. Cheung CS, Wong AW, Lui A, et al. Incidence of endophthalmitis and use of antibiotic prophylaxis after intravitreal injections. Ophthalmology. 2012; 119:1609–1614.
25. Meyer CH, Michels S, Rodrigues EB, et al. Incidence of rhegmatogenous retinal detachments after intravitreal antivascular endothelial factor injections. Acta Ophthalmol. 2011; 89:70–75.
26. Wei WB, Xu L, Jonas JB, et al. Subfoveal choroidal thickness: the Beijing Eye Study. Ophthalmology. 2013; 120:175–180.
27. Usui S, Ikuno Y, Akiba M, et al. Circadian changes in subfoveal choroidal thickness and the relationship with circulatory factors in healthy subjects. Invest Ophthalmol Vis Sci. 2012; 53:2300–2307.
28. Tan CS, Ouyang Y, Ruiz H, Sadda SR. Diurnal variation of choroidal thickness in normal, healthy subjects measured by spectral domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2012; 53:261–266.
29. Pryds A, Larsen M. Choroidal thickness following extrafoveal photodynamic treatment with verteporfin in patients with central serous chorioretinopathy. Acta Ophthalmol. 2012; 90:738–743.
30. Barteselli G, Chhablani J, El-Emam S, et al. Choroidal volume variations with age, axial length, and sex in healthy subjects: a three-dimensional analysis. Ophthalmology. 2012; 119:2572–2578.
31. Ouyang Y, Heussen FM, Mokwa N, et al. Spatial distribution of posterior pole choroidal thickness by spectral domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2011; 52:7019–7026.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download