Abstract
Purpose
The purpose of this study was to establish a set of normative data values for saccade movements using videonystagmography and to evaluate the effects of manual correction on this data.
Methods
We examined 25 healthy subjects (9 men and 16 women). All tests were carried out by one well-instructed physician. Errors such as the wrong detection of the inflection point, missing movement, and prediction occurred during some tests. Thus, the same physician manually corrected the data by deleting error data from row results.
Results
We established a set of normative data for horizontal saccade movements (amplitude size 15 and 30 degrees) for mean peak velocity, latency, and accuracy. Manual correction only impacted latency and accuracy at 30 degrees horizontal, which is likely related to possible errors during the test.
The main objectives of voluntary eye movements are either to position or maintain images of interest on the fovea, the small central retinal area of highest visual acuity. Saccades and smooth pursuit are controlled by different neural structures. These differing anatomical pathways include several cortical areas that mediate cognitive control of eye movements, in contrast to brainstem structures that are mainly concerned with the motor control of eye movements [1]. The analysis of eye movements has been shown to provide key contributions to the diagnosis of some neurodegenerative, hereditary, and metabolic disorders.
Videonystagmography (VNG) was designed to acquire video images of eye movements, especially in nystagmus, and is potentially useful to clinicians in otorhinolaryngology, neurology, and ophthalmology [2]. Traditionally, electronystagmography (ENG), which relies on the corneo-retinal potential to record eye movements, is considered the gold standard for evaluating dizzy patients [3]. In contrast to ENG, VNG records eye movements using digital video image technology employing infrared illumination to determine eye position. The use of VNG enables simultaneous subjective observation of eye movements together with objective data collection and analysis of eye movement waveforms via computer algorithms. VNG has the benefit of not requiring skin preparation or electrode application and wiring. Moreover, adjustments are seldom required as VNG does not depend on changes in corneo-retinal potential over time in contrast to ENG. However, VNG is unable to record eye movements when the eyes are closed [4].
To date, there has been no study reporting normative data of saccade movements obtained from an Asian patient population, including Koreans. The aim of this study was to establish a set of normative data of saccade movements obtained from 25 healthy Korean adults using VNG (SLVNG; SLMED, Seoul, Korea) and to evaluate the effects of manual correction on this data.
We examined 25 healthy volunteers (9 men and 16 women). The following subjects were included: those with no history of vertigo, balance problems, otologic problems, neurologic diseases, or ocular diseases, who were not on any medications. The inclusion criteria were assessed by history taking and basic examination for visual acuity and eye movement. All subjects were asked for their informed consent prior to entering this study. This study was reviewed and approved by the institutional review board of Kim's Eye Hospital, and all procedures conformed to the guidelines of the Declaration of Helsinki.
Eye movements were recorded with an infrared camera (resolution 640 × 480 pixels, frame rate 60 Hz) and displayed on a computer monitor (SLMED). In the saccade test, the patient was seated in the VNG chair, which was at a fixed, pre-calibrated distance from the VNG monitor, and asked to follow the movements of the object on the screen. A stimulus randomly appeared on the screen at the edge of the visual field in horizontal directions (amplitude size 15 and 30 degrees), and the patient was asked to follow the stimulus only with his eyes, keeping his head stable; eye movements were displayed and recorded and a computer analyzed the results.
Latency was defined as the delay between the onset of target movement and the initiation of eye movement. Accuracy was defined as the amplitude of the eye movement relative to the target. Velocity was defined as the time taken to complete the saccade once it was initiated.
All tests were carried out by one well-instructed physician. Errors such as wrong detection of the inflection point, missing movement, and prediction occurred during some tests (Fig 1). Thus, correction was required, and the same physician manually corrected the data by deleting error data from the row results.
The effect of manual correction was studied by comparing parameters before and after correction using the paired t-test (SPSS ver. 12.0, SPSS Inc, Chicago, IL, USA; p < 0.05).
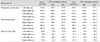
Our normative database contains over 25 saccadic eye movements from 25 normal subjects ranging from 21 to 38 years of age. Table 1 shows the mean peak velocity, latency, accuracy, and 95% confidence interval at 15 degree horizontal saccade movements. The results of 30 degree horizontal saccade movements are shown in Table 2. The effect of movement direction on saccade parameters was not significant, and there was no significant difference in the parameters between 15 and 30 degrees.
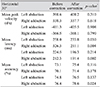
Analysis of the effects of manual correction in which one physician deleted extremely high or low data from row results showed significant differences only with respect to latency and accuracy at 30 degrees horizontal (Table 3).
The present study provides VNG-based normative data of saccade movements in Korean individuals. From the time the first accurate measurements of saccadic eye movements were obtained [5,6], several studies have reported peak velocity values in normal subjects, but these have usually been obtained from small numbers of young volunteers [6,7,8,9,10,11]. With respect to the effects of age on saccade eye movements, Abel et al. [12] found that there was no correlation, while Spooner et al. [13] reported that elderly subjects had lower peak velocity values.
Furthermore, Warabi et al. [14] reported that elderly people had slower large (40°) saccades than younger subjects, but that there were no differences at smaller saccade angles. In a study by Wilson et al. [15], time of day and age had weak but significant effects on saccade movements, but the effects of age only became apparent for large saccades (≥35°). Diurnal changes in performance have also been observed, with a tendency for slightly slower values in the early afternoon. Another study revealed that gender and education level did not influence eye movement metrics, and with age, the latency of leftward and vertical prosaccades and antisaccades increased, the velocity of upward prosaccades decreased, the gain of rightward and upward prosaccades diminished, and the error rate of antisaccades increased [1].
In the current study, we established a set of normative data values for saccade movements using VNG and identified the effects of manual correction on said data. To obtain reliable results from VNG, we acquired data in a clinical setting, and to reduce variability between inspectors, tests and manual correction were carried out by one well-instructed physician. None of our healthy subjects complained of any form of dizziness or other side effects during the test. Values obtained with our equipment are in agreement with those of previous studies (Table 4). There has been no study reporting normative data values for saccade movements obtained from an Asian population. In comparison with previous studies (Table 4), there was no significant difference in the velocity of eye movements based on ethnicity. Manual correction only impacted latency and accuracy at 30 degrees horizontal, which is likely related to possible errors during the test. For instance, the wrong detection of inflection point can cause delayed latency or decreased accuracy (Fig. 1A). Secondly, the infrared camera can miss the eye movement for various reasons such as thick eye make-up using eyeliner and false eyelashes or eyelid problems like ptosis, entropion, or small fissures (Fig. 1B). Thirdly, the participant's eye can move more quickly than the target when the patient predicts target movement (Fig. 1C). These errors can affect the latency and accuracy of VNG results.
There are some limitations to this study. First, the normative data were obtained from a small number of young healthy volunteers. Second, we did not attempt to investigate the effect of age or systemic diseases like diabetic mellitus on VNG results. Therefore, the normative data from this study may not be applicable to the elderly and patients with systemic diseases. Furthermore, nystagmography is more frequently used for the evaluation of eye movements in pediatric examinations, but there are no data or measurements from young children in this study. Future studies regarding the effects of specific conditions on VNG results are needed. Third, VNG might be very variable based upon the examiner and his or her level of expertise. Consequentially, its reliability needs to be confirmed for interpretation when using this normative data in clinics.
To adequately interpret a patient's VNG results, it is important to have access to up-to-date, reliable normative data. With this, VNG can reveal crucial information to enable the clinician to construct a management strategy to cure or aid patients with eye movement disorders.
Figures and Tables
 | Fig. 1Possible errors during the test. (A) Wrong detection of inflection point, (B) missing movement, and (C) prediction. |
References
1. Bonnet C, Hanuska J, Rusz J, et al. Horizontal and vertical eye movement metrics: what is important? Clin Neurophysiol. 2013; 124:2216–2229.
2. Casse G, Sauvage JP, Adenis JP, Robert PY. Videonystagmography to assess blinking. Graefes Arch Clin Exp Ophthalmol. 2007; 245:1789–1796.
3. McCaslin DL, Jacobson GP. Current role of the videonystagmography examination in the context of the multidimensional balance function test battery. Semin Hear. 2009; 30:242–252.
4. Naguib MB, Madian Y, Refaat M, et al. Characterisation and objective monitoring of balance disorders following head trauma, using videonystagmography. J Laryngol Otol. 2012; 126:26–33.
5. Robinson DA. The mechanics of human saccadic eye movement. J Physiol. 1964; 174:245–264.
6. Cook G, Stark L. The human eye-movement mechanism: experiments, modeling, and model testing. Arch Ophthalmol. 1968; 79:428–436.
7. Westheimer G. Mechanism of saccadic eye movements. AMA Arch Ophthalmol. 1954; 52:710–724.
8. Boghen D, Troost BT, Daroff RB, et al. Velocity characteristics of normal human saccades. Invest Ophthalmol. 1974; 13:619–623.
9. Baloh RW, Konrad HR, Sills AW, Honrubia V. The saccade velocity test. Neurology. 1975; 25:1071–1076.
10. Mims JL 3rd, Treff G. Saccadic velocities of horizontal rectus muscles in twenty-five normal humans. J Pediatr Ophthalmol Strabismus. 1982; 19:129–136.
11. Raab EL. Normal saccadic velocities. J Pediatr Ophthalmol Strabismus. 1985; 22:20–22.
12. Abel LA, Troost BT, Dell'Osso LF. The effects of age on normal saccadic characteristics and their variability. Vision Res. 1983; 23:33–37.
13. Spooner JW, Sakala SM, Baloh RW. Effect of aging on eye tracking. Arch Neurol. 1980; 37:575–576.
14. Warabi T, Kase M, Kato T. Effect of aging on the accuracy of visually guided saccadic eye movement. Ann Neurol. 1984; 16:449–454.
15. Wilson SJ, Glue P, Ball D, Nutt DJ. Saccadic eye movement parameters in normal subjects. Electroencephalogr Clin Neurophysiol. 1993; 86:69–74.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download