Abstract
Dengue fever is a viral disease that is transmitted by mosquitoes and affects humans. In rare cases, dengue fever can cause visual impairment, which usually occurs within 1 month after contracting dengue fever and ranges from mild blurring of vision to severe blindness. Visual impairment due to dengue fever can be detected through angiography, retinography, optical coherence tomography (OCT) imaging, electroretinography, event electroencephalography (visually evoked potentials), and visual field analysis. The purpose of this study is to report changes in the eye captured using fluorescein angiography, indocyanine green, and OCT in 3 cases of dengue fever visual impairment associated with consistent visual symptoms and similar retinochoroidopathic changes. The OCT results of the three patients with dengue fever showed thinning of the outer retinal layer and disruption of the inner segment/outer segment (IS/OS) junction. While thinning of the retina outer layer is an irreversible process, disruption of IS/OS junction is reported to be reversible. Follow-up examination of individuals with dengue fever and associated visual impairment should involve the use of OCT to evaluate visual acuity and visual field changes in patients with acute choroidal ischemia.
Dengue fever is a mosquito-transmitted viral disease that affects humans. There are 4 viral serotypes (DEN-1, DEN2, DEN-3, and DEN-4) of the genus Flavivirus (Flaviviridae family) commonly found in tropical and subtropical regions. Recently, dengue fever has become a major health concern due to increased prevalence, especially in Southeast Asia [1].
Dengue fever causes a variety of clinical symptoms such as headaches, self-limiting fever, and myalgia [2]. Hypotension leading to dengue shock syndrome and thrombocytopenia resulting in bleeding manifestations may also occur. Ocular manifestations with visual impairment are not commonly found in dengue fever but have been more frequently described in the literature over the last few years than in the past [3]. Visual impairment ranges from mild blurring of the vision to severe blindness and usually occurs within the first month after contracting dengue fever. Retinal examination in these individuals has revealed macular and retina hemorrhage, peripapillary hemorrhage, diffuse retina edema, Roth's spot, vitreous cells, macular yellow spots, and optic disc swelling [4].
Visual impairment by dengue fever can be detected by ophthalmological exams including angiography, retinography, optical coherence tomography (OCT) imaging, electroretinography, event electroencephalography (visual evoked potential), and visual field analysis [5]. While the pathophysiologic mechanisms involving the retina and choroidal changes in dengue fever are not completely understood, they are thought to be a result of complex immune-mediated processes rather than a direct viral invasion [6]. There have been several reports regarding retinal and choroidal changes by immune complexes, such as acquired immunodeficiency syndrome-related retinochoroidopathy and Systemic lupus erythematosus-related choroidopathy, but the pathogenesis of these conditions is also not known.
Changes by choroidal nonperfusion or retinal pigment epithelium (RPE) inflammation in white dot syndrome have been reported. Herein, we report changes and features of fluorescein angiography, indocyanine green (ICG), and OCT in 3 cases of dengue fever that demonstrated consistent visual symptoms and similar retinochoroidopathy changes.
A 40-year-old woman presented with a 1-year history of afterimages in both eyes after having Dengue fever. Her best-corrected visual acuity (BCVA) was 20 / 20 in both eyes, and her intraocular pressure was normal. Slit examination revealed no abnormal findings. Retina examination revealed hemorrhaging in the retina inner layer and RPE atrophy in both eyes. Fluorescein angiography demonstrated fluorescence blockage by retinal hemorrhage. ICG showed late hypofluorescence due to choroidal filling disorder, and infrared (IR) imagery showed hypofluorescence that included both foveae. OCT imagery revealed disruption of the inner segment/outer segment junction (IS/OS) junction and outer retina thinning at the hypofluorescence site of the IR imagery (Figs. 1 and 2).
A 40-year-old woman presented with an upper visual field defect in the left eye after contracting dengue fever 1 month prior. The right eye was normal. BCVA was 20 / 30 in the left eye, and no other abnormal findings were found in the anterior segment. Retina examination revealed RPE atrophy in the left eye. Fluorescein angiography (FA) of the left eye showed a patch choroidal perfusion delay and a window defect due to RPE atrophy. IR imagery demonstrated hypofluorescence in the superior nasal area including the fovea. OCT imagery revealed disruption of the IS/OS junction and outer retina thinning at the hypofluorescence site of the IR imagery.
A 29-year-old woman treated for dengue fever 1 year prior presented with a visual field defect in the left eye. All symptoms and test were normal in the right eye. The anterior segment was normal. Retina examination showed RPE mottling in the left eye. FA revealed a faint window defect in the left eye, and IR imagery revealed hypofluorescence in the fovea area. OCT imagery revealed disruption of the IS/OS junction and outer retina thinning at the hypofluorescence site of the IR imagery.
The incidence of ocular manifestations in dengue fever is rare and usually occurs during the acute phase of disease. The main fundus changes are macula and retinal hemorrhages, peripapillary hemorrhage, Roth's spot, diffuse retinal edema, vitreous cells, blurring of the optic disc margin, serous retinal detachment, choroidal effusions, and nonspecific maculopathy [4]. It has been reported that approximately 10% of all dengue fever patients suffer from maculopathy with central vision loss [7]. In our study, all 3 patients had observable RPE atrophy on the FA; disruption of the IS/OS junction and outer retina thinning were also observed on the IR imagery.
The pathogenesis of ocular manifestations of dengue infection is not completely understood, but is thought to be caused by either a direct viral invasion or an immune-mediated reaction [6]. The mean interval between the onset of visual symptoms and the systemic manifestation of dengue fever reported by Wen et al. [4] was 7.26 days (range, 2 to 15 days) and that reported by Lim et al. [8] was 6.8 days (range, 6 to 7 days), which suggests an immune-mediated process rather than a direct viral infection. This immune-mediated process likely comprises a complex series initiated by direct viral infection. Viral invasion of endothelial cells, dendritic cells, monocytes, and hepatocytes cause apoptosis and cellular dysfunction, which may be followed by a transient aberrant immune response, resulting in CD4/CD8 ratio inversion and cytokine overproduction [9,10]. In addition, overproduction of interleukin-6 triggers the formation of autoantibodies against platelets and endothelial cells, resulting in further immune-mediated damage [9-11] and leading to endothelial dysfunction, platelet destruction, and consumptive coagulopathy. Ultimately, the aggressive immune response may cause increased capillary permeability, plasma leakage, and hemorrhagic diathesis. This hemorrhage bleeding tendency can occur in both the retina and choroid causing retina hemorrhage and choroidal circulatory disorders.
The outer retina layer is fed by choroidal circulation making it vulnerable to ischemic diseases. In our study, we noted a decrease in the outer retina layer thickness and a choroidal circulation disorder in all three cases. In case 1, choriocapillary nonperfusion was seen in the ICG images. Similar choroidal changes can be seen in Wegner's granulomatosis, where ischemic atrophy of the outer retina by choroidal ischemia has been reported [12]. This similarity also supports the possibility that an immune-mediated complex is the cause of the choroidal changes in dengue fever rather than direct viral invasion.
Henoch-Schonlein purpura, essential cryoglobulinemia, serum sickness vasculitis, lupus vasculitis, and hepatitis B microscopic polyarteritis have been reported as vasculitis diseases caused by immune complexes that mostly invade the capillaries, venules, and arterioles. In cases of large vessels immune complex-mediated vasculitis, complexes are removed quickly by MPS cells, thus causing minimal damage to the vessels.
South Korea is not an endemic region for dengue fever, and therefore, acute stage patients are rarely observed. Frequently used test such as the hemagglutination inhibition test and immunoglobin G or immunoglobin M enzyme immunoassays were not performed in our study and this is a limitation for this study. In the future, complementary measurements in the serum of dengue fever patients will be needed to further understand the disease process.
In conclusion, thinning of the retina outer layer is irreversible. Conversely, IS/OS junction disruption is reported to be reversible, so follow-up studies using OCT are necessary to evaluate visual acuity and visual field changes in acute choroidal ischemia in order to improve vision in dengue fever patients with ocular manifestations.
Figures and Tables
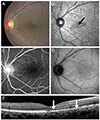 | Fig. 1Left eye of case 1 at presentation. (A) Color fundus photo showing retinal dot lesions around the macula. (B) Red-free fundus photograph revealing hypofluorescence in the macula (black arrow). (C) Fluorescence blockage by retinal hemorrhage can be seen in the fluorescein angiography. (D) Indocyanine green angiography shows retinal hemorrhage around the macula with hypofluorescence in the late phase. (E) Spectral domain optical coherence tomography images show foveal disruption of the photoreceptor layer (between the white arrows), whereas the external limiting membrane is continuous. |
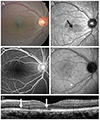 | Fig. 2Right eye of case 1 at presentation. (A) Color fundus photo showing retinal dot lesions around the macula. (B) Red-free fundus photograph revealing hypofluorescence in the macula (black arrow). (C) Fluorescence blockage by retinal hemorrhage can be seen in the fluorescein angiography. (D) Indocyanine green angiography shows retinal hemorrhage around the macula with hypofluorescence in the late phase. (E) Spectral domain optical coherence tomography images show foveal disruption of the photoreceptor layer (between the white arrows), whereas the external limiting membrane is continuous. |
References
1. Gubler DJ. The global emergence/resurgence of arboviral diseases as public health problems. Arch Med Res. 2002; 33:330–342.
2. Richardson S. Ocular symptoms and complications observed in dengue. Trans Am Ophthalmol Soc. 1933; 31:450–477.
3. Cruz-Villegas V, Berrocal AM, Davis JL. Bilateral choroidal effusions associated with dengue fever. Retina. 2003; 23:576–578.
4. Wen KH, Sheu MM, Chung CB, et al. The ocular fundus findings in dengue fever. Gaoxiong Yi Xue Ke Xue Za Zhi. 1989; 5:24–30.
5. Chia A, Luu CD, Mathur R, et al. Electrophysiological findings in patients with dengue-related maculopathy. Arch Ophthalmol. 2006; 124:1421–1426.
6. Morens DM. Antibody-dependent enhancement of infection and the pathogenesis of viral disease. Clin Infect Dis. 1994; 19:500–512.
7. Su DH, Bacsal K, Chee SP, et al. Prevalence of dengue maculopathy in patients hospitalized for dengue fever. Ophthalmology. 2007; 114:1743–1747.
8. Lim WK, Mathur R, Koh A, et al. Ocular manifestations of dengue fever. Ophthalmology. 2004; 111:2057–2064.
9. Kurane I, Rothman AL, Livingston PG, et al. Immunopathologic mechanisms of dengue hemorrhagic fever and dengue shock syndrome. Arch Virol Suppl. 1994; 9:59–64.
10. Lei HY, Yeh TM, Liu HS, et al. Immunopathogenesis of dengue virus infection. J Biomed Sci. 2001; 8:377–388.
11. Lin CF, Lei HY, Shiau AL, et al. Antibodies from dengue patient sera cross-react with endothelial cells and induce damage. J Med Virol. 2003; 69:82–90.
12. Iida T, Spaide RF, Kantor J. Retinal and choroidal arterial occlusion in Wegener's granulomatosis. Am J Ophthalmol. 2002; 133:151–152.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download