Abstract
A myxoma is a benign tumor found in the heart and in various soft tissues; however, a corneal myxoma is rare. A mucinous mass of unknown etiology was observed on the left cornea of a 32-year-old male patient. We performed deep anterior lamellar keratoplasty using acellular corneal tissue and concurrent amniotic membrane transplantation. Hematoxylin and eosin staining revealed vacuolation of the parenchyma and myxoid change in the corneal tissue that occurred in the anterior half of the corneal parenchyma. We identified a myxoid stroma by Alcian blue staining and observed collagen fibers with denatured stroma by Masson trichrome staining. The patient's visual acuity improved from light perception to 20 / 200, and the intraocular pressure remained within the normal range for one year after surgery. The transplanted cornea survived successfully with well-maintained transparency, and recurrence was not observed one year after surgery.
A myxoma is a benign tumor found in the heart and in various soft tissues [1]. A myxoma is infrequently observed in ophthalmic structures, but has been described in the conjunctiva, orbit, eyelid [2]. Corneal myxoma is rare, and only 11 cases have been described in the literature [3-8]. Although further long-term studies are required for confirmation, deep anterior lamellar keratoplasty (DALK) with acellular corneal tissue may be considered one of the best surgical options for high-risk corneas with a healthy endothelium. Acellular corneal tissue may help to increase the supply of donated corneas and can be readily accessible in emergency situations or remote areas because it can be stored at room temperature for up to one year [9,10]. Here, we describe the treatment of an 8-mm gelatinous mass via DALK using albumin-preserved acellular corneal tissue (VisionGraft Sterile Cornea; TBI/Tissue Banks International, Baltimore, MD, USA).
A 32-year-old male patient was examined for vision loss and the presence of a mass in the left eye in December 2010. He had been examined at other hospitals for chronic open-angle glaucoma of the left eye that had persisted since 2006. Small blisters of the cornea began to appear in October 2007; these blisters progressed gradually and eventually invaded the entire cornea. Before the appearance of the lesion, no other treatments, such as laser therapy or phototherapy had been applied, with the exception of eye drops (Alphagan-P; Allergan, Irvine, CA, USA). When the patient first visited our clinic, the visual acuity of his left eye was light perception, and the intraocular pressure (IOP) measured by a Tono-Pen (Tono-Pen AVIVA, Reichert, NY, USA) was 43 mmHg. Upon, slit-lamp examination, a 8.7 × 7-mm gelatinous mass and blisters covering the entire cornea with new vessels at the 6 o'clock position were observed. The entire corneal thickness was 1.39 mm, the depth of the degenerated lesion was 0.89 mm, and the residual corneal tissue was 0.50 mm, as measured by anterior optical tomography (Visante optical coherence tomography; Carl Zeiss Meditec, Dublin, CA, USA) (Fig. 1).
The host cornea was trephinated to a level of 70% to 80% of its thickness using the 8.0-mm Hessburg-Barron disposable vacuum trephine (Katena Instruments, Denville, NJ, USA). A blunt spatula was used to separate the overlying corneal tissue along the plane of dissection. The anterior lamellar stroma (about 80% in thickness) was removed using Vannas scissors. The residual stroma was removed layer-by-layer with repeated dissections until Descemet's membrane was exposed. The acellular corneal tissue, with a diameter of 8.5 mm, was sutured in place using 32 interrupted stitches of 10-0 nylon. We performed permanent amniotic membrane grafting with a temporary amniotic membrane patch to minimize epithelization delay. The temporary amniotic membrane and stitches were removed one week after the surgery, and the corneal epithelial defects remained one month after surgery. However, these defects had healed by three months after surgery. IOP remained within the normal range, and visual acuity increased to 20 / 200. One year after treatment, no recurrence was observed (Fig. 2).
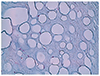
The size of the lesion was 8.7 × 7 mm, and it consisted of gelatinous white tissue with an irregular surface. Under the corneal epithelium, myxoid tissue was observed, with a severely vacuolar degenerated stroma beneath the myxoid tissue. The myxoid tissue was positive on Alcian blue staining (pH 2.5), and a few scattered fibroblasts were observed on hematoxylin and eosin (H&E) staining (Figs. 3, 4, and 5). The lesion appeared to be a loose, hypocellular, minimal collagen-depositional, glycosaminoglycan-rich myxoid mass in the subepithelial anterior stroma. Moreover, the central site of the lesion exhibited vacuolar change beneath the myxoid tissue (Fig. 6). The spindle cells were negative for amyloid (Congo red) and the vacuoles were negative for chondrocytes (Toluidine blue).
Myxomas are derived from abnormal fibroblasts that secrete excess mucopolysaccharides instead of producing mature collagen fibers [1,11]. Corneal myxomas are considered to be a secondary reactive process [12], and the tumor cells have been suggested to derive from embryonic mesenchymal cells with a multipotential character [13]. A primary myxoma in the cornea is considered to be rare and a secondary corneal myxoma is a reactive process in someone who has had prior corneal disease or injury inducing chronic corneal edema [3]. Corneal myxomas tend to form anteriorly beneath the epithelium and arise in previously inflamed or injured corneas [12]. Generally, corneal myxomas show spindle-shaped cells within a loose stroma (H&E), and the myxoid stroma stains strongly with Alcian blue, indicating high glycosaminoglycan content [5]. Clinically, the differential diagnoses considered were Salzmann's nodular degeneration, allergic hypertrophia, a foreign body reaction, pannus, corneal keloid, amyloid deposition, dermoid tumor, and corneal squamous cell carcinoma [3].
The patient in this study was being treated with Alphagan-P for chronic open-angle glaucoma in a different hospital. However, there have been no reports that factors, such as glaucoma or glaucomatous agents, induce secondary corneal myxoma. In this case, the myxoma is assumed to be a primary corneal myxoma, because the growing myxoma induced increased IOP, and IOP returned to the normal range when the myxoma was removed.
This case was similar to a corneal myxoma in the gross and pathological findings on H&E, Alcian blue, and Masson trichrome staining, but had a lower cell density than other myxomas reported to date [4,5,8]. Moreover, the accompanying vacuolar changes in this case were different from those of past myxomas. Contact lens-induced epithelial microcysts or corneal pseudocysts have been previously reported [14-16], but microcysts were limited in the epithelium and those previous cases differed from this case in the size and number of cysts. Contact lens-induced epithelial microcysts were induced by decreased aerobic metabolic activity due to chronic hypoxia in extended lens wear [16]. Similarly, the vacuolar changes likely resulted from the accumulation of metabolites such as lactic acid in the stroma, because the air supply to the cornea was blocked for a long time by the gelatinous tissue. However more research is necessary to identify the factors that can induce vacuolar changes in the corneal stroma.
DALK is used to remove pathological corneal stroma from Descemet's membrane and involves transplantation of the stroma from a donor's cornea. The advantage of this procedure is that it preserves the recipient's corneal inner layer and reduces the occurrence of transplant rejection and failure [17]. Recently, DALK using glycerol-preserved corneal tissue was reported to reduce allogeneic immune rejection [10].
In this case, the patient's Descemet's membrane was intact, but the size of the corneal lesion was 8 mm, which indicated a greater possibility of rejection and involvement of the deep stroma. Therefore, we considered DALK using an acellular cornea. We used albumin-preserved acellular corneal tissue from which the cells were removed by gamma irradiation so that the graft was less likely to be rejected. Gamma irradiation has been shown to kill bacteria, fungi, and viruses. Previous studies have shown that it can also inactivate prions while maintaining the integrity of human albumin, which may be a distinct advantage when compared with other forms of corneal storage available today [9,18-20]. Furthermore, acellular corneal tissue can be stored at room temperature for one year [9].
Amniotic membrane is known to decrease surface inflammation and scarring in addition to facilitating epithelialization [21]. We performed amniotic membrane transplantation for the purpose of improving the microenvironment of limbal epithelial stem cells [22]. In conclusion, we experienced an 8-mm myxoma in the anterior half of the corneal stroma, accompanied by vacuolation, and we treated it via lamellar keratoplasty with acellular corneal tissue.
Figures and Tables
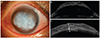 | Fig. 1(A) A preoperative anterior segment photograph of the left eye shows a jelly-like corneal degeneration (8.7 × 7 mm). (B,C) Anterior optical tomography (Visante OCT) shows a 1.39-mm full-thickness cornea and a 0.89-mm-deep area of corneal degeneration. |
 | Fig. 2(A) A postoperative anterior segment photograph taken 90 days after deep anterior lamellar keratoplasty of the left eye showing a completely healed cornea. (B) Almost corneal epithelial defects had healed by three months after surgery. |
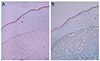 | Fig. 3The lesion is covered by degenerated squamous epithelium. The underlying stroma shows marked myxoid and vacuolar degeneration, with positive Alcian blue staining. A few fibroblast-like spindle cells are scattered in the degenerated stroma. (A) Hematoxylin and eosin, ×40; (B) Alcian blue stain, ×40. |
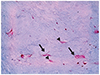 | Fig. 4A high-magnification view of the stroma shows uninucleated (arrowhead) and multinucleated (arrow) spindle cells scattered throughout the myxoid stroma (Alcian blue stain, ×200). |
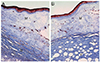 | Fig. 6(A) The periphery of the lesion showing a loose, hypocellular, minimal collagen-depositional, glycosaminoglycan-rich lesion in the subepithelial anterior stroma (M), in sharp contrast to the normal deep stroma (N). (B) The central site of the lesion showing vacuolar changes in the deep stroma (V). Masson trichrome stain, ×100. |
References
1. Bulkley BH, Hutchins GM. Atrial myxomas: a fifty year review. Am Heart J. 1979; 97:639–643.
2. Horie Y, Ikawa S, Okamoto I, et al. Myxoma of the conjunctiva: a case report and a review of the literature. Jpn J Ophthalmol. 1995; 39:77–82.
3. Wollensak G, Green WR, Seiler T. Corneal myxoma. Jpn J Ophthalmol. 2002; 46:193–197.
4. Hansen LH, Prause JU, Ehlers N, Heegaard S. Primary corneal myxoma. Acta Ophthalmol Scand. 2004; 82:224–227.
5. Chang HJ. Superficial corneal growth. JAMA. 2011; 305:2467–2468.
6. Soong T, Soong V, Salvi SM, et al. Primary corneal myxoma. Cornea. 2008; 27:1186–1188.
7. Khan AO, Al-Katan H, Al-Gehedan S. Infantile corneal myxoma. J AAPOS. 2008; 12:207–209.
8. Robinson JW, Brownstein S, Mintsioulis G. Corneal myxoma arising in a patient with repeated phototherapeutic keratectomies. Cornea. 2006; 25:1111–1114.
9. Daoud YJ, Smith R, Smith T, et al. The intraoperative impression and postoperative outcomes of gamma-irradiated corneas in corneal and glaucoma patch surgery. Cornea. 2011; 30:1387–1391.
10. Li J, Yu L, Deng Z, et al. Deep anterior lamellar keratoplasty using acellular corneal tissue for prevention of allograft rejection in high-risk corneas. Am J Ophthalmol. 2011; 152:762–770.
11. Enzinger FM. Intramuscular myxoma: a review and follow-up study of 34 cases. Am J Clin Pathol. 1965; 43:104–113.
12. Perez-Grossmann RA, Mesias LA, Contreras F, Spencer WH. Solitary corneal myxoma. Cornea. 1997; 16:498–500.
13. Stout AP. Myxoma, the tumor of primitive mesenchyme. Ann Surg. 1948; 127:706–719.
14. Wilbanks GA, Boerner S, Smith D, Rootman DS. Giant pseudocyst formation associated with chronic corneal edema. Cornea. 1997; 16:224–226.
15. Margo CE, Mosteller MW. Corneal pseudocyst following acute hydrops. Br J Ophthalmol. 1987; 71:359–360.
16. Holden BA, Sweeney DF, Vannas A, et al. Effects of long-term extended contact lens wear on the human cornea. Invest Ophthalmol Vis Sci. 1985; 26:1489–1501.
17. Tsubota K, Kaido M, Monden Y, et al. A new surgical technique for deep lamellar keratoplasty with single running suture adjustment. Am J Ophthalmol. 1998; 126:1–8.
18. Erickson GA, Landgraf JG, Wessman SJ, et al. Detection and elimination of adventitious agents in continuous cell lines. Dev Biol Stand. 1989; 70:59–66.
19. House C, House JA, Yedloutschnig RJ. Inactivation of viral agents in bovine serum by gamma irradiation. Can J Microbiol. 1990; 36:737–740.
20. Miekka SI, Forng RY, Rohwer RG, et al. Inactivation of viral and prion pathogens by gamma-irradiation under conditions that maintain the integrity of human albumin. Vox Sang. 2003; 84:36–44.
21. Shimazaki J, Yang HY, Tsubota K. Amniotic membrane transplantation for ocular surface reconstruction in patients with chemical and thermal burns. Ophthalmology. 1997; 104:2068–2076.
22. Kim JS, Kim JC, Hahn TW, Park WC. Amniotic membrane transplantation in infectious corneal ulcer. Cornea. 2001; 20:720–726.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download