This article has been
cited by other articles in ScienceCentral.
Abstract
Purpose
In Asian countries, laser iridotomy for the treatment of angle-closure glaucoma is a common cause of bullous keratopathy, which may be associated with a shallow anterior chamber and dark iris pigmentation in Asians. Several cases of corneal decompensation after argon laser iridotomy have been reported. In the present study, we evaluated the harmful effects of argon laser iridotomy on the corneal endothelium.
Methods
Argon laser iridotomy was performed on the right eyes of pigmented rabbits. Changes in corneal thickness and endothelial cell density after laser iridotomy were evaluated. Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) was performed for assessment of corneal endothelial cell apoptosis. Combined staining with alizarin red and trypan blue, as well as a live/dead cell assay, were performed for evaluation of damage to the corneal endothelium induced by laser iridotomy.
Results
Corneal thickness did not change immediately after laser iridotomy; however, a significant increase was observed 24 hours after iridotomy (p = 0.001). The endothelial cell density of laser-treated eyes four days after laser iridotomy was significantly decreased compared with control eyes (p < 0.001). TUNEL staining showed many TUNEL-positive cells in the corneal endothelium and corneal stroma. No endothelial trypan blue-stained cell nuclei were observed after laser iridotomy; however, several large endothelial cells with damaged membrane integrity were observed. The live/dead cell assay clearly showed a large number of dead cells stained red in several areas throughout the entire corneal button 24 hours after iridotomy.
Conclusions
Argon laser iridotomy induces corneal endothelial cell apoptosis in pigmented rabbit eyes, resulting in decreased endothelial cell density.
Go to :

Keywords: Apoptosis, Argon laser iridotomy, Cornea, Corneal endothelium
Although the average corneal endothelial cell density in neonates is 3,500 to 4,000 cells/mm
2, cell density continues to decrease throughout life, with an average cell loss of 0.3% to 0.6% per year [
1-
3]. As a result, the average density in adults is decreased to 1,400 to 2,500 cells/mm
2 [
2,
3], indicating that endothelial cell proliferation is not significant in humans. Although the cause of endothelial cell loss over time has not been fully elucidated, apoptosis or necrosis caused by light-induced oxidative damage may play a role in the mechanism [
4,
5]. Normal age-related cell loss does not affect the barrier or pump functions of the endothelium. However, if endothelial cell loss occurred at a more rapid rate than normal, the endothelium could not maintain its function, resulting in bullous keratopathy and loss of vision.
Bullous keratopathy is a common indication for keratoplasty. Corneal decompensation may occur due to several causes, including surgical trauma, previous corneal transplantation, systemic diseases such as diabetes, topical or systemic medications, toxic anterior segment syndrome, and endothelial dystrophy [
6-
13]. In Asian countries, laser iridotomy for treatment of angle-closure glaucoma is a common cause of bullous keratopathy, which may be associated with the shallow anterior chamber and dark iris pigmentation in Asians [
14,
15]. Several cases of corneal decompensation after argon laser iridotomy have been reported [
16-
19]. However, to the best of our knowledge, no previous animal studies have assessed the harmful effects of argon laser iridotomy on the corneal endothelium. In this study, we evaluated the effects of argon laser iridotomy on the corneal endothelium using pigmented rabbit eyes, which are similar to the brown eyes in Asians.
Materials and Methods
Eyes of pigmented rabbits are similar to brown eyes in Asians, and argon laser iridotomy could not be performed in New Zealand white rabbits because of poor absorption of thermal energy; thus, pigmented rabbits were used in this study. Use and treatment of the rabbits conformed to the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research.
Argon laser peripheral iridotomy was performed on the right eyes of 20 pigmented rabbits under general anesthesia with an intramuscular injection of ketamine hydrochloride (40 mg/kg of body weight). The left eye was used as a control. A green ophthalmic laser machine (OcuLight GL; Iridex, Mountain View, CA, USA) was used, and the same laser settings (power, 500 mW; duration, 200 ms; spot size, 75 µm; count, 400 to 500 times) were maintained throughout this study. A peripheral iridotomy was performed on each of the four quadrants (superior temporal, superior nasal, inferior temporal, and inferior nasal) using an Abraham iridotomy contact lens. The iridotomy incision size was similar to that used in clinical practice (
Fig. 1). No topical or systemic medication for reducing inflammation after laser iridotomy was used. Central corneal thickness was measured using an ultrasound corneal pachymeter (BV International, Clermont-Ferrand, France) before, immediately after, and 24 hours after iridotomy. Corneal thickness was measured three times and the mean values were analyzed.
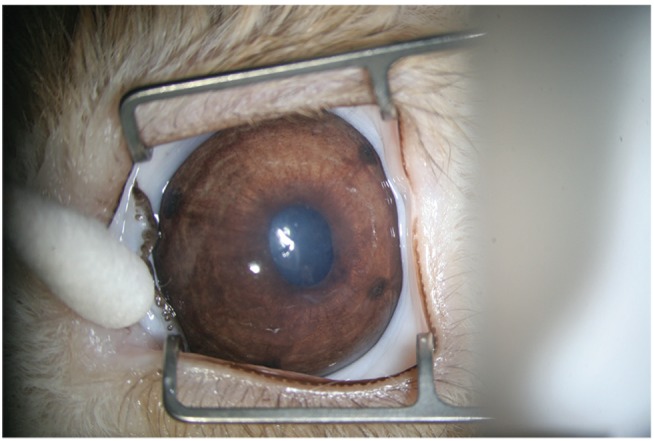 | Fig. 1A rabbit eye after argon laser peripheral iridotomy in each of four quadrants (superior temporal, superior nasal, inferior temporal, and inferior nasal) using an Abraham iridotomy contact lens. 
|
Rabbits were anesthetized with an intramuscular injection of ketamine hydrochloride and were humanely killed by intracardiac injection of 2% lidocaine hydrochloride. Eyes were enucleated and frozen immediately after sacrifice in optimum cutting temperature compound (Tissue-Tek; Miles Laboratories, Elkhart, IN, USA) using liquid nitrogen. Frozen tissue blocks were stored at -85℃ until they were sectioned. Central corneal sections (7 µm thick) were cut using a cryostat at -20℃ and were placed on silanized microscope slides (DAKO, Carpinteria, CA, USA). Some tissue sections were stained with hematoxylin and eosin for histological observation, and others were fixed in cold acetone at -20C for 2 minutes for terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL). TUNEL assay for the detection of apoptotic cells was performed in four rabbits 24 hours after laser iridotomy using the ApopTag Red In Situ Apoptosis Detection Kit (Cat no. S7165; Chemicon International, Temecula, CA, USA). DAPI was used for nuclei counterstaining.
To evaluate corneal endothelial integrity, dual staining of corneal endothelium with trypan blue and alizarin red was performed in eight rabbits. Isolated corneas were placed endothelial side up in a Teflon corneal cup, and a 7.5-mm-sized corneal button was cut from the center using a surgical corneal punch. Trypan blue was added dropwise to cover the endothelium of the corneal disc and allowed to react for 2 minutes. The corneal disc was briefly rinsed twice in normal saline, drained to remove excess saline, and then replaced in the corneal cup. The endothelial layer was then covered with alizarin red (0.4%, pH 4.2) for 3 minutes and rinsed twice in saline after discarding the staining reagent. After the staining procedure, corneal discs were fixed in glutaraldehyde solution for 15 minutes. The corneal disc was mounted endothelial side up on a microscope slide having a central cavity to accommodate the thickness of the corneal button.
To assess the harmful effects of argon laser iridotomy, cell viability was investigated in eight rabbits using a live/dead viability/cytotoxicity kit (Molecular Probes, Eugene, OR, USA). Staining was performed according to the manufacturer's instructions. Live cells distinguished by the presence of ubiquitous intracellular esterase activity were stained green, whereas dead cells with damaged membranes were stained red.
Endothelial cells stained with alizarin red four days after laser iridotomy were counted in three consecutive light microscopic fields under high magnification (×400) by a blinded observer, and then the cell density per mm2 was calculated. Cell density was compared between laser-treated and control eyes in four rabbits.
The SPSS ver. 10.1 (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. Paired t-test was used for comparison between the mean corneal thickness before and after laser iridotomy. For comparison of cell density, a non-parametric Mann-Whitney test was used because samples were not sufficient for a parametric test. A p-value <0.05 was considered statistically significant.
Go to :

Results
Argon laser iridotomy was performed successfully in 20 pigmented rabbits. The mean central corneal thickness (n = 10) was 369.2 µm (±22.8 µm) before laser iridotomy, 372.0 µm (±25.8 µm) immediately after laser iridotomy and 405.4 µm (±25.4 µm) 24 hours after laser iridotomy. Although corneal thickness did not differ significantly before and immediately after iridotomy (
p = 0.276), the mean corneal thickness significantly increased 24 hours after iridotomy compared with before laser iridotomy (
p = 0.001) (
Fig. 2).
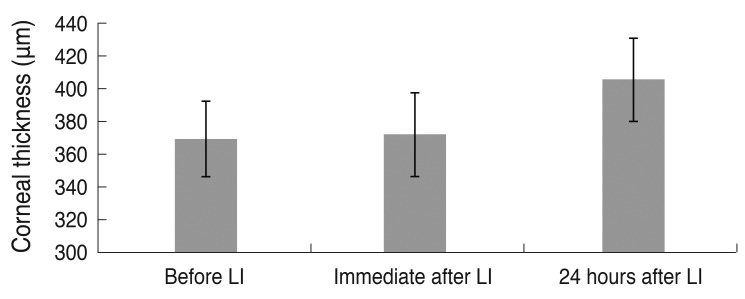 | Fig. 2Change in mean central corneal thickness after argon laser iridotomy (LI). Although corneal thickness did not differ significantly before and immediately after iridotomy (p = 0.276), it showed a significant increase 24 hours after iridotomy compared with before LI (p = 0.001, n = 10). 
|
In normal rabbit corneas, TUNEL-positive cells were not detected in the corneal stroma or corneal endothelium (n = 4) (
Fig. 3A). However, 24 hours after argon laser iridotomy, many TUNEL-positive cells were observed in the corneal endothelium as well as in the corneal stroma (n = 4) (
Fig. 3B), indicating that argon laser iridotomy induced apoptosis in stromal keratocytes and corneal endothelial cells.
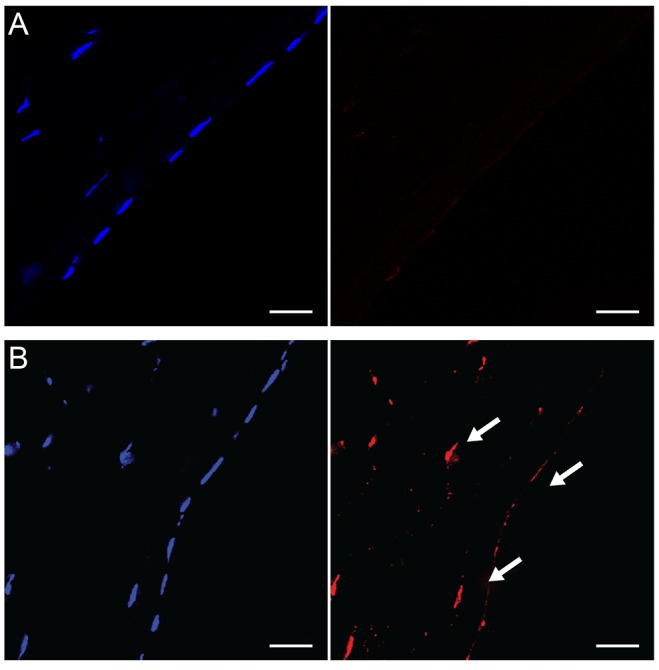 | Fig. 3(A) In normal rabbit corneas, terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL)-positive cells were not detected in the corneal stroma or corneal endothelium. (B) Twenty-four hours after argon laser iridotomy, many TUNEL-positive cells (arrows) were observed in the corneal endothelium as well as in the corneal stroma. TUNEL staining, bar = 20 µm. 
|
In dual staining of the corneal endothelium with trypan blue and alizarin red 24 hours after iridotomy (n = 4), intercellular borders of endothelial cells were clearly stained with alizarin red, and several large endothelial cells with damaged membrane integrity were observed (
Fig. 4A). However, no endothelial cells with dark blue nuclei stained by trypan blue were observed (
Fig. 4B). Four days after iridotomy, damaged endothelial cells were no longer found, and a normal mosaic pattern was observed (n = 4) (
Fig. 4C). The number of endothelial cells was counted in the central 0.04 mm
2 in four pigmented rabbits four days after laser iridotomy and was used to calculate the cell density per mm
2. The mean cell density was 4,375.0 cells/mm
2 (±276.1 cells/mm
2), which was significantly lower than 5,387.5 cells/mm
2 (±194.2 cells/mm
2) in the normal control (
p < 0.001).
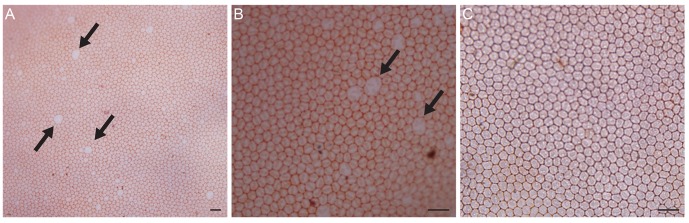 | Fig. 4(A) Twenty-four hours after iridotomy, intercellular borders of endothelial cells were clearly stained with alizarin red, and several large endothelial cells with damaged membrane integrity (arrows) were observed. (B) Endothelial cells with trypan blue-stained nuclei were not observed. (C) Four days after iridotomy, damaged endothelial cells were no longer found, and a normal mosaic pattern was observed. Dual staining of trypan blue and alizarin red, bar = 40 µm. 
|
The live/dead cell assay showed that almost all endothelial cells of normal corneas were viable and stained green (
Fig. 5A). However, one day after iridotomy, many dead, red-stained cells were observed throughout the entire corneal button (
Fig. 5B). The mean percentage of live cells in three consecutive microscopic fields under high magnification (×400) was decreased to 78.9% (±4.15%) (n = 4,
p < 0.001) (
Fig. 6). Four days after iridotomy, dead cells were no longer observed (n = 4) (
Fig. 5C).
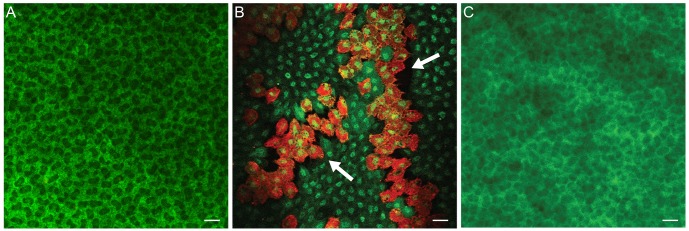 | Fig. 5(A) Almost all endothelial cells of normal cornea were viable and stained green. (B) One day after iridotomy, many dead cells stained red were observed in several areas throughout the corneal button. (C) Four days after iridotomy, dead cells were no longer observed. Live/dead cell assay, bar = 20 µm. 
|
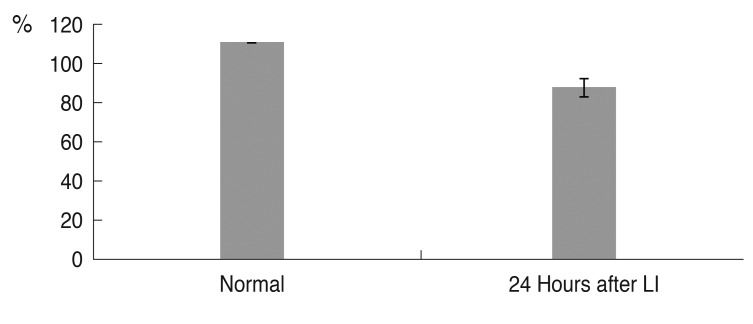 | Fig. 6The mean percentage of live cells in the corneal endothelium. Although 100% of corneal endothelial cells in the normal cornea were alive, 24 hours after laser iridotomy (LI), the percentage of live cells was 78.9% (n = 4, p < 0.001). 
|
Go to :

Discussion
Over the past decade, lamellar keratoplasty has supplanted penetrating keratoplasty (PK) as new surgical procedures have been introduced. Endothelial keratoplasty has become the procedure of choice in patients with endothelial disease because many of the ocular surface and wound integrity problems associated with PK can be avoided, and faster visual rehabilitation is possible [
20]. Deep anterior lamellar keratoplasty has many advantages over PK, including the absence of endothelial rejection, less steroid exposure, less long-term endothelial loss, and less distortion of angle anatomy; therefore, it is considered a good alternative to PK in patients whose endothelium is not compromised [
21,
22]. In addition, a single-donor cornea can be used in two patients needing lamellar keratoplasty. However, many Asian countries, including Japan and South Korea, have a shortage of donor corneas; a large number of visually compromised patients with corneal disease have to wait a long time for a chance to receive a corneal transplantation. In these countries, prevention is very important in order to avoid the need for corneal transplantation, and novel methods to protect against damage to the cornea, especially endothelial cells, should be investigated along with the evolution of corneal transplantation procedures. That is the reason the present animal study was undertaken.
Argon laser iridotomy is known to induce bullous keratopathy by damaging endothelial cells [
14-
16] and is a growing problem in Asian countries because angle-closure glaucoma is more common in Asia than in Western countries. Angle-closure glaucoma patients usually have a shallow anterior chamber and appear to be more vulnerable to laser iridotomy. Although direct focal laser injury to the endothelium is one mechanism of corneal damage, other factors such as thermal damage from an increase in aqueous humor temperature, mechanical shock waves and turbulent flow of aqueous humor through iridotomy sites, and anterior chamber inflammation may be involved in the pathological process [
14,
16,
18,
23]. Lim et al. [
19] reported 14 cases of inferior corneal decompensation after laser peripheral iridotomy in the superior iris. In these cases, direct focal injury or shock waves and turbulent flow of aqueous humor through iridotomy sites did not cause corneal decompensation. Instead, anterior chamber inflammation may have played a major role in the pathogenesis of bullous keratopathy. Sagoo et al. [
24] demonstrated that inflammatory cytokines such as interleukin-1, interferon-γ, and tumor necrosis factor induce corneal endothelium apoptosis through the production of nitric oxide. Therefore, in the present study, the corneal center was evaluated separate from the peripheral iridotomy site in order to detect the endothelial damage induced by an indirect mechanism such as anterior chamber inflammation.
We assessed changes in corneal thickness and endothelial cell density after argon laser iridotomy. Although corneal thickness did not change considerably immediately after laser iridotomy, a significant increase was observed 24 hours after iridotomy (p = 0.001). Acute anterior chamber inflammation associated with peripheral laser iridotomy might induce rapid cell degeneration in the corneal center, resulting in temporary endothelial cell dysfunction and corneal edema 24 hours after iridotomy. However, the endothelial cell loss usually occurs in a focal manner and is repaired by sliding and rearranging neighboring cells. Therefore, dead endothelial cells were no longer observed four days after iridotomy. However, the endothelial cell density became significantly lower in laser-treated eyes compared to control eyes.
TUNEL staining, which was performed 24 hours after laser iridotomy to determine whether argon laser iridotomy induced corneal endothelial cell apoptosis, showed many TUNEL-positive cells in the corneal endothelium as well as in the corneal stroma. Laser iridotomy induced corneal endothelial cell apoptosis, and, as a result, endothelial cell density decreased. To the best of our knowledge, this is the first study demonstrating that argon laser iridotomy induces corneal endothelial cell apoptosis and keratocytes in an animal model. In the present study, anterior chamber inflammation after laser iridotomy was not treated with topical corticosteroids in order to maximize the effect of anterior chamber inflammation on the corneal endothelium. However, if acute inflammation is adequately controlled, the damage to corneal endothelial cells might be reduced. Therefore, we are planning a study to evaluate the protective effects of topical corticosteroids on the corneal endothelium after laser iridotomy.
Combined staining of corneal endothelium with alizarin red and trypan blue has been used to delineate damaged from undamaged cells [
25,
26]. In contrast to healthy cells, damaged cells become permeable to trypan blue and show deep blue staining of their nuclei. However, in this study, there were no endothelial cells whose nuclei were stained with trypan blue after laser iridotomy, even though several large endothelial cells with damaged membrane integrity were observed. The live/dead cell assay clearly showed many dead cells stained red in several areas throughout the corneal button 24 hours after iridotomy. This difference may be due to the sensitivity of the two staining methods. The live/dead cell assay provides a two-color fluorescence staining, coloring the cytoplasm of damaged endothelial cells bright red and the nuclei of dead corneal endothelial cells dark blue. The contrast between live and dead cells appears more prominent in a live/dead cell assay. In addition, the vital dye staining with alizarin red and trypan blue has no reproducible staining protocol, and obtaining reliable staining results by replicating the protocols previously described is difficult [
27]. Therefore, the live/dead cell assay is a more sensitive tool for the detection of damaged corneal endothelial cells after laser iridotomy than combined staining with alizarin red and trypan blue.
This study had several limitations. First, only one iridotomy was performed in each of the four quadrants, and thus, the total laser energy was greater than in actual clinical practice. Although animal studies should be conducted in a manner similar to actual clinical practice, rabbits have a deeper anterior chamber than patients with angle-closure glaucoma. Therefore, we hypothesized that more laser energy would be necessary to induce the harmful effects of laser iridotomy in rabbit eyes. Therefore, four sites of peripheral iridotomy were chosen in each treated eye.
This is a preliminary study to evaluate the harmful effects of argon laser iridotomy on the corneal endothelium. Further studies are needed to evaluate the effect of laser iridotomy depending on the total energy or total count used for iridotomy and to determine how to minimize injury to the corneal endothelium. The other limitation is that rabbits were used in this study. Although the rabbit animal model has several advantages, rabbit corneal endothelial cells are different than human corneal endothelial cells with respect to cell proliferation. Rabbit corneal endothelial cells can proliferate in vivo. Therefore, this animal model is not appropriate for evaluating the long-term effects of laser iridotomy on human corneal endothelial cells. However, this rabbit animal model appears useful for evaluating the damage to corneal endothelial cells over a short period of time.
In conclusion, we demonstrated that argon laser iridotomy induced apoptosis of corneal endothelial cells in pigmented rabbit eyes, resulting in decreased endothelial cell density. Argon laser iridotomy showed a definite effect on the function and cell density of corneal endothelium in this pigmented rabbit eye model. Compared with conventional combined staining with alizarin red and trypan blue, the live/dead assay more sensitively detected the corneal endothelium damage induced by laser iridotomy.
Go to :

Acknowledgements
This study was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2012R1A1A2042054), Seoul, South Korea. This study was supported in part by Alumni of Department of Ophthalmology, Korea University College of Medicine.
Go to :

Notes
Go to :

References
1. Murphy C, Alvarado J, Juster R, Maglio M. Prenatal and postnatal cellularity of the human corneal endothelium. A quantitative histologic study. Invest Ophthalmol Vis Sci. 1984; 25:312–322. PMID:
6698749.
2. Joyce NC. Proliferative capacity of the corneal endothelium. Prog Retin Eye Res. 2003; 22:359–389. PMID:
12852491.

3. Klyce SD. Corneal physiology. In : Foster CS, Azar DT, Dohlman CH, editors. Smolin and Thoft's the cornea. Philadelphia: Lippincott Williams & Wilkins;2004. p. 37–58.
4. Cho KS, Lee EH, Choi JS, Joo CK. Reactive oxygen species-induced apoptosis and necrosis in bovine corneal endothelial cells. Invest Ophthalmol Vis Sci. 1999; 40:911–919. PMID:
10102288.
5. Koh SW, Waschek JA. Corneal endothelial cell survival in organ cultures under acute oxidative stress: effect of VIP. Invest Ophthalmol Vis Sci. 2000; 41:4085–4092. PMID:
11095600.
6. Taylor DM, Atlas BF, Romanchuk KG, Stern AL. Pseudophakic bullous keratopathy. Ophthalmology. 1983; 90:19–24. PMID:
6338434.

7. Bigar F, Witmer R. Corneal endothelial changes in primary acute angle-closure glaucoma. Ophthalmology. 1982; 89:596–599. PMID:
7122040.

8. Bourne WM. Cellular changes in transplanted human corneas. Cornea. 2001; 20:560–569. PMID:
11473153.

9. Schultz RO, Matsuda M, Yee RW, et al. Corneal endothelial changes in type I and type II diabetes mellitus. Am J Ophthalmol. 1984; 98:401–410. PMID:
6486211.

10. Konowal A, Morrison JC, Brown SV, et al. Irreversible corneal decompensation in patients treated with topical dorzolamide. Am J Ophthalmol. 1999; 127:403–406. PMID:
10218692.

11. Hughes B, Feiz V, Flynn SB, Brodsky MC. Reversible amantadine-induced corneal edema in an adolescent. Cornea. 2004; 23:823–824. PMID:
15502485.

12. Duffy RE, Brown SE, Caldwell KL, et al. An epidemic of corneal destruction caused by plasma gas sterilization: the Toxic Cell Destruction Syndrome Investigative Team. Arch Ophthalmol. 2000; 118:1167–1176. PMID:
10980761.
13. Adamis AP, Filatov V, Tripathi BJ, Tripathi RC. Fuchs' endothelial dystrophy of the cornea. Surv Ophthalmol. 1993; 38:149–168. PMID:
8235998.

14. Ang LP, Higashihara H, Sotozono C, et al. Argon laser iridotomy-induced bullous keratopathy a growing problem in Japan. Br J Ophthalmol. 2007; 91:1613–1615. PMID:
17567658.
15. Shimazaki J, Amano S, Uno T, et al. National survey on bullous keratopathy in Japan. Cornea. 2007; 26:274–278. PMID:
17413952.

16. Schwartz AL, Martin NF, Weber PA. Corneal decompensation after argon laser iridectomy. Arch Ophthalmol. 1988; 106:1572–1574. PMID:
3056353.

17. Jeng S, Lee JS, Huang SC. Corneal decompensation after argon laser iridectomy: a delayed complication. Ophthalmic Surg. 1991; 22:565–569. PMID:
1961612.
18. Wilhelmus KR. Corneal edema following argon laser iridotomy. Ophthalmic Surg. 1992; 23:533–537. PMID:
1508483.

19. Lim LS, Ho CL, Ang LP, et al. Inferior corneal decompensation following laser peripheral iridotomy in the superior iris. Am J Ophthalmol. 2006; 142:166–168. PMID:
16815272.

20. Price MO, Price FW Jr. Endothelial keratoplasty: a review. Clin Experiment Ophthalmol. 2010; 38:128–140. PMID:
20398103.
21. Sutphin JE, Goins KM, Wagoner MD. Deep anterior lamellar keratoplasty: when should it replace penetrating keratoplasty? Am J Ophthalmol. 2009; 148:629–631. PMID:
19878753.

22. Han DC, Mehta JS, Por YM, et al. Comparison of outcomes of lamellar keratoplasty and penetrating keratoplasty in keratoconus. Am J Ophthalmol. 2009; 148:744–751. PMID:
19589495.

23. Yamamoto Y, Uno T, Shisida K, et al. Demonstration of aqueous streaming through a laser iridotomy window against the corneal endothelium. Arch Ophthalmol. 2006; 124:387–393. PMID:
16534059.

24. Sagoo P, Chan G, Larkin DF, George AJ. Inflammatory cytokines induce apoptosis of corneal endothelium through nitric oxide. Invest Ophthalmol Vis Sci. 2004; 45:3964–3973. PMID:
15505043.

25. Spence DJ, Peyman GA. A new technique for the vital staining of the corneal endothelium. Invest Ophthalmol. 1976; 15:1000–1002. PMID:
62728.
26. Sperling S. Combined staining of corneal endothelium by alizarine red and trypane blue. Acta Ophthalmol (Copenh). 1977; 55:573–580. PMID:
70953.

27. Park S, Fong AG, Cho H, et al. Protocol for vital dye staining of corneal endothelial cells. Cornea. 2012; 31:1476–1479. PMID:
22531436.

Go to :








 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download