Abstract
Purpose
Methods
Results
Conclusions
Figures and Tables
 | Fig. 1The typical multifocal visual evoked potential signal is divided into two parts (windows) according to implicit time. The signal window between 0 to 200 msec and the noise window between 300 to 500 msec. Signal to noise ratio is calculated based on this division of the waveform. |
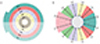 | Fig. 2The five allocated hemi-rings and their corresponding fellows in both hemispheres (A). The allocated six sectors and their corresponding fellows in both hemispheres (B). SR = superior hemi-ring; IR = inferior hemi-ring; SS = superior sector; IS = inferior sector. |
 | Fig. 3The 58 segments of the right visual field. The field is divided into two identical hemifields across the horizontal meridian; each segment has a similar correspondent in the opposite hemifield. (A) Signal to noise ratio (SNR) value is calculated for each segment. The average SNR of wedge sectors (B) and semicircular sectors; peripheral and central sectors (C,D) are calculated to compare their values to fellow corresponding sectors on the opposite hemifield. |
 | Fig. 4Receiver operating characteristic analysis for the glaucoma suspect group by multifocal visual evoked potential testing in the glaucoma suspect group. |
 | Fig. 5Receiver operating characteristic analysis for the glaucoma group by multifocal visual evoked potential testing in the glaucoma suspect group. |
 | Fig. 6Box plot of signal to noise ratio (SNR) values of multifocal visual evoked potential in normal, glaucoma suspect, and glaucoma groups. There was a significant difference between the mean values among the three study groups. |
 | Fig. 7Box plot of the difference in signal to noise ratio (SNR) values of multifocal visual evoked potential in normal, glaucoma suspect, and glaucoma groups. There was a significant difference between the mean values among the three study groups. |
Table 1

Age distribution of all subjects in the three study groups. There was no significant statistical difference in age among groups that could have affected the data (p = 0.964 for the normal group, 0.964 for the glaucoma suspect group, and 0.810 for the glaucoma group). This indicates that age did not have a significant on the data.
*One way ANOVA test. Significant when p < 0.05.
Table 2

Only one pair of sectors (SS5-IS5) showed a statistically significant difference in signal to noise ratio values. All other tested sectors and hemi-rings were not statistically significant.
N = number of eyes; M = mean difference in signal to noise ratio values; SD = standard deviation; SS = superior sector; IS = inferior sector; SR = superior hemi-ring; IR = inferior hemi-ring.
*Paired t-test, significant when p < 0.05.
Table 3

Four pairs of sectors (4 / 6) showed a statistically significant difference in signal to noise ratio values, while only one hemi-ring (1 / 5) showed a statistically significant difference. All other tested sectors and hemi-rings were not statistically significant.
N = number of eyes; M = mean difference in signal to noise ratio values; SD = standard deviation; SS = superior sector; IS = inferior sector; SR = superior hemi-ring; IR = inferior hemi-ring.
*Paired t-test, significant when p < 0.05.
Table 4

All pairs of sectors (6 / 6) and hemi-rings (5 / 5) showed a statistically significant signal to noise ratio difference.
N = number of eyes; M = mean difference in signal to noise ratio values; SD = standard deviation; SS = superior sector; IS = inferior sector; SR = superior hemi-ring; IR = inferior hemi-ring.
*Paired t-test, significant when p < 0.05.
Table 6

Comparison of signal to noise ratio values in each sector and hemi-ring between the three groups. There was a highly significant statistical difference (p < 0.001) between groups in all tested sectors and hemi-rings.
N = number of eyes; M = mean difference in signal to noise ratio values; SD = standard deviation; SS = superior sector; IS = inferior sector; SR = superior hemi-ring; IR = inferior hemi-ring.
*One way ANOVA test, significant when p < 0.05.
Table 8

Significant differences between groups in the Intersector comparison (superior and inferior). Most of the tested sectors were significant (7 / 11). N = number of eyes; M = mean difference in signal to noise ratio values; SD = standard deviation; SS = superior sector; IS = inferior sector; SR = superior hemi-ring; IR = inferior hemi-ring.
*One way ANOVA test, significant when p < 0.05.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


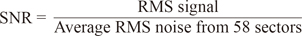





 XML Download
XML Download