Abstract
Purpose
To evaluate the effects of a bimatoprost/timolol fixed combination (BTFC) and a latanoprost/timolol fixed combination (LTFC) on diurnal intraocular pressure (IOP) and anterior ocular parameters in healthy subjects.
Methods
We enrolled 58 healthy subjects in this prospective clinical study. Thirty subjects were treated with BTFC and 28 subjects were treated with LTFC. IOP was measured every 2 hours except from 01:00 and 05:00. Axial length, corneal curvature, and anterior chamber depth were obtained using the IOL master at baseline and 24 hours later. Adverse events were assessed by patient interview and by slit lamp examination.
Results
The largest difference in IOP between treated and untreated eyes 8 hours after instillation was 1.67 mmHg in the BTFC group (p < 0.001). The largest difference in IOP between treated and untreated eyes 10 hours after instillation was 1.93 mmHg in the LTFC group (p < 0.001). For anterior ocular parameters such as axial length, corneal curvature, anterior chamber depth at baseline and 24 hours after instillation, there were no significant differences between the baseline and 24-hour values in either the BTFC or LTFC group. The most frequently occurring adverse event was conjunctival hyperemia, which was found in 33.3% (n = 10) of the BTFC group and 25.0% (n = 7) of the LTFC group (p = 0.486).
Glaucoma is one of the leading causes of irreversible visual field loss and blindness [1,2]. Increased intraocular pressure (IOP) is an important factor in glaucoma development and progression. A large number of studies have shown that a reduction in IOP in the early phase is crucial for glaucoma treatment [3-5]. The Early Manifest Glaucoma Trial showed that a 1 mmHg reduction in IOP decreases the glaucoma progression risk by 10% [3]. The American Academy of Ophthalmology's Preferred Practice Guidelines recommend a 20% to 30% reduction in IOP from the initial IOP. The Ocular Hypertension Treatment Study, in which IOP reduction lowered the risk of glaucoma development in ocular hypertension patients, targeted a 20% IOP reduction [4,5].
The fixed combination of latanoprost and timolol (LTFC; Xalacom; Pharmacia, Kalamazoo, MI, USA; Pfizer, New York, NY, USA) was the first prostaglandin analogue/beta-blocker fixed combination available in ophthalmology. Bimatoprost/timolol fixed combination (BTFC; Ganfort, Allergan, Irvine, CA, USA) is another fixed combination drug that combines bimatoprost 0.03% and timolol 0.5%. The IOP-lowering effects and the association of its individual components have been reported [6-17]. In recent years, usage of such a fixed combination drug has increased due to superior drug effects and patient compliance.
Treatment with a fixed combination may increase compliance by simplifying the regimen and reducing the washout effect of previously instilled eye drops. Furthermore, fixed combination eye drops can lead to better compliance for long-term glaucoma management because of the ease of once-daily administration and avoidance of excessive preservative-related ocular toxicity associated with multiple instillations [18].
Determining the IOP responses is very important in glaucoma treatment, but precisely determining the IOP responses in each individual is very difficult. Diurnal variation in IOP is an important factor in glaucoma treatment. Several studies have revealed that diurnal IOP is different between individuals [19,20]. IOP changes involve both true pharmacological effects and spontaneous IOP fluctuations. The latter are reported to be greater in glaucoma patients than in healthy (normal) subjects [21,22].
A monocular trial is one of the methods for identifying the effect of an eye drop. Many studies have been performed to evaluate the efficacy of monocular drug trials [23-27]. At certain times after application in one eye, the effect of the eye drop is estimated from the difference in IOP between the instilled eye and the other eye [23,25,26]. However, there are various methods depending on the drug, patient, and clinician. For instance, an assessment of the effect of a single drop on diurnal IOP or a comparison of IOP at baseline and at several days or weeks after treatment has been used in clinical conditions. To date, there has been no report on the effects of an ocular trial in healthy subjects as a reference. The purpose of this study was to evaluate the effects of LTFC and BTFC on diurnal IOP and anterior ocular parameters in healthy subjects.
This study was a prospective clinical study that included new patients who visited the glaucoma department of Kangbuk Samsung Hospital, Seoul, Korea, from September 2009 to February 2011. This study was performed in adherence with the Declaration of Helsinki. Approval was received from the institutional review board and ethics committee of Kangbuk Samsung Hospital in Seoul, Korea. We enrolled non-glaucoma subjects. The inclusion criteria for healthy subjects are summarized in Table 1. Exclusion criteria included active ocular disease, abnormally low or high blood pressure or pulse rate, contraindications or sensitivity to any component of the study treatments, use of other ocular medications or other therapies that might have a substantial effect on IOP, and a history of ocular surgery. Women who were not using an effective means of contraception or who were pregnant or nursing were excluded. Subjects who were taking medication with side effects of altering IOP, such as steroid, β-blocker, systemic diuretics (carbonic anhydrase inhibitor), or antipsychotic medications, were excluded. The subjects were randomly treated with either LTFC ophthalmic solution (Xalacom) or BTFC ophthalmic solution (Ganfort).
IOP was measured by slit lamp using a Goldmann applanation tonometer. A single examiner measured IOP in all patients using the same applanator. At the time of the IOP measurement, three consecutive measurements of each eye were obtained, and the mean of the three measurements was analyzed. Neither the examiner nor the healthy subjects in the two groups knew which group was being tested.
First, we assessed the effects of one drop each of LTFC (n = 28) and BTFC (n = 30) on diurnal IOP. We randomly chose one eye of a normal subject and applied one drop of the LTFC or BTFC in the morning (between 8:00 a.m. and 8:30 a.m.). We measured the IOP ten times a day (baseline-before instillation, 09:00, 11:00, 13:00, 15:00, 17:00, 19:00, 21:00, 23:00, and 07:00). The patients slept without any disturbance between 01:00 and 05:00. The difference in diurnal IOP between the instilled eye and the untreated fellow eye was evaluated by paired t-test. Furthermore, the difference in diurnal IOP in the instilled eye between the LTFC group and the BTFC group was evaluated by independent samples t-test.
Second, we evaluated the effects of the LTFC or BTFC therapy on anterior ocular parameters. Axial length, corneal curvature, and anterior chamber depth were obtained using an IOL master (Carl Zeiss Meditec, Jena, Germany) at baseline and 24 hours. The difference in the anterior ocular parameters between before and after drug application was evaluated by paired t-test.
Adverse events were assessed by patient interview including foreign-body sensation, itchiness, stinging and by slit lamp examination including conjunctival hyperemia, and superficial punctuate epitheliopathy. The difference in the adverse events in the instilled eye between the LTFC group and the BTFC group was evaluated by chi-square test. Baseline demographics were compared between the LTFC and the BTFC groups using independent sample t-test for continuous variable and chi-square test for categorical variables, as appropriate. All data were analyzed using the SPSS ver. 18.0 (SPSS Inc., Chicago, IL, USA). A p-value less than 0.05 was considered statistically significant.
We investigated 58 patients (116 eyes) of which 23 (39.7%) were men and 35 (60.3%) were women. The mean age was 66.67 ± 11.48 years (range, 35 to 91 years). The demographic characteristics of the subjects in the two groups are summarized in Table 2. The mean ages and sexes of the BTFC and LTFC groups were not statistically different (independent sample t-test, p = 0.824; chi-square test, p = 0.120; respectively) (Table 2). The difference in baseline IOP between the BTFC group and LTFC group was statistically significant (Table 2). The baseline IOP between instilled and untreated eyes of each group was not statistically different (paired samples t-test, p = 0.110 for BTFC group, p = 0.445 for LTFC group) (Tables 3 and 4).
The measurements of diurnal IOP after instillation of one drop of the BTFC are summarized in Table 3. Diurnal IOPs after instillation of one drop of BTFC between the instilled and untreated eyes of normal subjects are shown in Fig. 1. The largest difference in IOP between the instilled and untreated eyes was observed 8 hours after instillation (1.67 mmHg) (p < 0.001) (Table 3).
The measurements of diurnal IOP after instillation of one drop of the LTFC are summarized in Table 4. Diurnal IOPs after instillation of one drop of LTFC between the instilled and untreated eyes of normal subjects are shown in Fig. 2. The largest difference in IOP between the instilled and untreated eyes was observed 10 hours after instillation (1.93 mmHg) (p < 0.001).
The IOP reductions from baseline for the BTFC and LTFC groups are summarized in Table 5. In the BTFC group, the largest reduction in IOP from baseline was observed 6 hours after instillation (2.57 ± 2.47 mmHg). In the LTFC group, the largest reduction in IOP from baseline was observed 12 hours after instillation (2.36 ± 3.37 mmHg).
The effects of BTFC and LTFC on axial length, corneal curvature, and anterior chamber depth at baseline and 24 hours after instillation are summarized in Tables 6 and 7. There were no significant differences between the baseline and 24-hour values.
There was no significant difference in adverse events between the BTFC and LTFC groups (Table 8). The most frequently occurring adverse event was conjunctival hyperemia, which was found in 33.3% (n = 10) of the BTFC group and 25.0% (n = 7) of the LTFC group (p = 0.486).
The results of our study demonstrate that both BTFC and LTFC produce a significant reduction in IOP from baseline without changing the anterior ocular parameters. We measured the IOP to obtain daytime IOP variation after one application of BTFC or LTFC. There were significant differences in the IOP after instillation. The largest difference in IOP was observed 8 hours after instillation in the BTFC group (13.6% reduction) and 10 hours after instillation in the LTFC group (17.8% reduction).
In our study, BTFC and LTFC showed different action times. The difference in IOP between instilled and untreated eyes was maintained for 10 hours after instillation in the BTFC group. Twenty-four hours after instillation, the IOP was lower than baseline, but there was no significant difference between the instilled eye and the untreated eye in the BTFC group. The difference in IOP between the instilled and untreated eyes was maintained for 20 hours after instillation in the LTFC group. In the LTFC group, the IOP at 24 hours after instillation was significantly lower than baseline.
In our study, there were differences in the IOP response between the BTFC and LTFC groups. Especially, the BTFC group showed a shorter duration of the IOP-lowering effect compared with LTFC group. The IOP-lowering effects of bimatoprost monotherapy have been reported [28-30]. BTFC showed a therapeutic advantage over the individual components [15,16]. Timolol eye drops twice a day are recommended, but one drop of timolol was used in this study. Therefore, IOP-lowering effects may be affected by bimatoprost and timolol. The reduced timolol effect may be one reason, however the LTFC contains the same ingredients (timolol). The stability of the drug combination may be a reason for the observed effect, but this alone can not explain the difference. The longer the treatment, the greater the IOP-lowering effects. The reason for the shorter duration of the bimatoprost IOP-lowering effects compared to effect of latanoprost may be that latanoprost is pharmacologically classified as a prostaglandin analog, but bimatoprost is classified as a prostamide because it is an amide rather than an ester compound [31]. However, we were unable to test this hypothesis based on the results of the present study. Furthermore, latanoprost acid has a higher affinity for the prostanoid FP receptor than bimatoprost acid [32], and a higher receptor affinity should result in a longer drug effect [29,33].
Cho et al. [34] reported that after instillation with one drop of brimonidine/timolol fixed combination, the largest difference in IOP between treated and untreated eyes 6 hours after instillation was 1.7 mmHg (p = 0.011). Differences in IOP between instilled eyes and untreated eyes first appeared 4 hours after instillation and were maintained for 14 hours after instillation. However, Cho et al. [34] did not measure IOP later than 14 hours after instillation. In the present study, the largest difference in IOP between treated and untreated eyes 8 hours after instillation was 1.67 mmHg in the BTFC group (p < 0.001). Differences in IOP between instilled eyes and untreated eyes first appeared 4 hours after instillation and were maintained for 14 hours after instillation in the BTFC group. The largest difference in IOP between treated and untreated eyes 10 hours after instillation was 1.93 mmHg in the LTFC group (p < 0.001). Differences in IOP between instilled eyes and untreated eyes first appeared 4 hours after instillation and were maintained for 20 hours after instillation in the LTFC group. The difference in results among the three drugs is thought to be due to the difference in the drug characteristics of prostaglandin analogs, prostamide and α2-adrenoceptor agonist (brimonidine).
In this study, BTFC showed a mean IOP fluctuation of 1.1 ± 0.71 mmHg, and LTFC showed a mean IOP fluctuation of 1.21 ± 0.88 mmHg. These ranges for the 24-hour pressure for the fixed combinations were lower than those reported in past studies [13,17,35]. These different results may be due to single instillation of the eye drop. It seems that treatment for several weeks or months shows different results.
For anterior ocular parameters such as axial length, corneal curvature, and anterior chamber depth at baseline and 24 hours after instillation, there were no significant differences in either the BTFC or LTFC group. The BTFC and LTFC groups showed a significant reduction in IOP from baseline without a change in the anterior ocular parameters. In a previous study, the axial length of primary open-angle glaucoma patients with latanoprost or bimatoprost treatment for approximately 2.37 years (more than 1 year) was not different from that of the control group [36]. However, the anterior chamber depth with prostaglandin analogue treatment was lower than that of the control group [36]. In another study, the anterior chamber depth with glaucoma or ocular hypertension patients decreased after 1 month of latanoprost treatment [37]. The different results observed in the present study may be associated with the single instillation of the eye drop, small population, and/or short follow-up duration. A large population-based long-term follow-up study will be needed to verify our results.
Conjunctival hyperemia is one of the most common local ocular side effects of prostaglandin analogue use [14,17,30,38]. In our study, conjunctival hyperemia occurred in both the BTFC and LTFC groups, but we did not identify any significant difference between the two groups (Table 8), possibly due to the small study population and short follow-up duration. Based on several studies, initial conjunctival hyperemia appears to be more severe with bimatoprost (in BTFC) than latanoprost [14,17,30]. Bimatoprost is already in its active form in the conjunctiva at the moment of application [39]. Latanoprost is absorbed through the cornea, and the isopropyl ester prodrug is hydrolyzed in the cornea to become the biologically active latanoprost acid [39,40]. For this reason, the conjunctival hyperemia is milder with latanoprost than with bimatoprost [14,17,30]. However, prostaglandin-associated conjunctival hyperemia is mild and decreases with time [30,41]. Therefore, we did not grade conjunctival hyperemia because it is difficult to assess the drug side effects with only a 1-drop instillation.
The exact monocular trial duration and methods for evaluation of drug effect have not yet been determined. There are various methods to do so depending on the drug, patient, and clinician. For instance, assessments of the IOP-lowering effects at several days or weeks after treatment have been used. And assessments of the IOP-lowering effects at several hours after one-drop treatment have also been used. We instilled one eye drop in one eye and used the untreated fellow eye as the control. With longer treatment duration with a prostaglandin analogue, the effect on IOP reduction would likely be increased. However, timolol can have a crossover effect that causes reduced IOP in the untreated fellow eye due to systemic absorption [42]. A monocular trial showed that timolol treatment can cause therapeutic lowering of IOP in the untreated fellow eye as a result of instillation of the medication in the treated eye (i.e., a contralateral effect) [42]. In the previous literature, timolol was shown to have a crossover effect that caused a lowering of IOP by about 1.5 mmHg in the untreated eye [43,44]. In this case, long-term treatment can interfere with the exact assessments of the IOP-lowing effect. We attempted to rule out the effects of systemic absorption by directly applying an eye drop and measuring the IOP for a relatively short period (24 hours) during hospitalization. Diurnal IOP was measured after instillation of only one eye drop, and the cul de sac was compressed for 5 minutes to avoid absorption through the lacrimal gland and to block the crossover effect of timolol maleate to the untreated eye. Therefore, an assessment of the effects of a one-time instillation of an eye drop on diurnal IOP seems to be useful for comparison of IOP at baseline and at several days to weeks after treatment in a clinical condition.
Our study design was the same as the method used in outpatient clinics for evaluating the reaction of normal subjects. The IOP-lowering effect of the eye drops was measured by evaluating the reaction of normal subjects. This study suggests that measurement of the IOP at 8 to 10 hours after instillation of a fixed combination drug will illustrate the most effective medication response.
Our study had some limitations. First, subjects of our study were normal healthy patients, not glaucoma patients. However, glaucoma patients are not ideal for monocular treatment protocols intended to study the intra-individual difference in drug efficacy because of the poor symmetry of IOP fluctuation between eyes. Therefore, we used normal subjects for this study. Second, our study was limited by its short duration. Twenty-four hours was not a sufficient duration for evaluating changes in IOP level or assessing the presence or absence of many potentially adverse events. Therefore, another trial with a longer term treatment is necessary to address these issues. Furthermore, our study did not provide information about IOP during the night. It is well known that the risk of glaucoma progression is increased, at least in some cases, by the fact that IOP may be higher during the night [29,45,46]. In our study, no IOP measurement between 1 a.m. to 6 a.m. was performed in order to minimize the influence of sleep disturbance on the true pattern of blood pressure or IOP variation.
In conclusion, BTFC and LTFC provided a significant reduction in IOP from baseline without changing anterior ocular parameters. BTFC and LTFC showed different diurnal IOP profile changes. The largest difference in IOP was seen 8 hours after instillation in BTFC and 10 hours after instillation in LTFC. Our results can act as a proper reference in a monocular trial for clinical assessment. Further research is necessary to clarify the effects of various fixed combination drugs in various conditions.
Figures and Tables
 | Fig. 1Diurnal intraocular pressure (IOP) measurement after instillation of one drop of fixed combination bimatoprost/timolol between instilled and untreated eyes of normal subjects. |
 | Fig. 2Diurnal intraocular pressure (IOP) measurement after instillation of one drop of fixed combination of latanoprost and timolol between instilled and untreated eyes of normal subjects. |
Table 1
Inclusion criteria for healthy subject

IOP = intraocular pressure.
*All visual fields were assessed using full threshold white-on-white Humphrey standard program 30-2. A technically acceptable visual field was considered abnormal if p < 5% for the corrected pattern standard deviation or if the glaucoma hemifield test was outside normal limits by STATPAC 2.
Table 3
Diurnal intraocular pressure (mmHg) after instillation of one drop of the fixed combination of bimatoprost/timolol and differences in intraocular pressure between instilled and untreated eyes of normal subjects
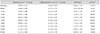
Table 4
Diurnal intraocular pressure (mmHg) after instillation of one drop of the fixed combination of latanoprost/timolol and differences in intraocular pressure between instilled and untreated eyes of normal subjects
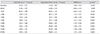
Table 6
The effects of the fixed combination bimatoprost/timolol on axial length, corneal curvature, anterior chamber depth over time at baseline and 24 hours later after instillation

Acknowledgments
This study was supported by a grant of the Korea Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (A101727).
References
1. Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006; 90:262–267.
2. Klaver CC, Wolfs RC, Vingerling JR, et al. Age-specific prevalence and causes of blindness and visual impairment in an older population: the Rotterdam Study. Arch Ophthalmol. 1998; 116:653–658.
3. Heijl A, Leske MC, Bengtsson B, et al. Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch Ophthalmol. 2002; 120:1268–1279.
4. Kass MA, Heuer DK, Higginbotham EJ, et al. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002; 120:701–713.
5. Fremont AM, Lee PP, Mangione CM, et al. Patterns of care for open-angle glaucoma in managed care. Arch Ophthalmol. 2003; 121:777–783.
6. Diestelhorst M, Almegard B. Comparison of two fixed combinations of latanoprost and timolol in open-angle glaucoma. Graefes Arch Clin Exp Ophthalmol. 1998; 236:577–581.
7. Larsson LI. Effect on intraocular pressure during 24 hours after repeated administration of the fixed combination of latanoprost 0.005% and timolol 0.5% in patients with ocular hypertension. J Glaucoma. 2001; 10:109–114.
8. Larsson LI. The effect on diurnal intraocular pressure of the fixed combination of latanoprost 0.005% and timolol 0.5% in patients with ocular hypertension. Acta Ophthalmol Scand. 2001; 79:125–128.
9. Higginbotham EJ, Feldman R, Stiles M, et al. Latanoprost and timolol combination therapy vs monotherapy: one-year randomized trial. Arch Ophthalmol. 2002; 120:915–922.
10. Pfeiffer N. European Latanoprost Fixed Combination Study Group. A comparison of the fixed combination of latanoprost and timolol with its individual components. Graefes Arch Clin Exp Ophthalmol. 2002; 240:893–899.
11. Diestelhorst M, Larsson LI. European Latanoprost Fixed Combination Study Group. A 12 week study comparing the fixed combination of latanoprost and timolol with the concomitant use of the individual components in patients with open angle glaucoma and ocular hypertension. Br J Ophthalmol. 2004; 88:199–203.
12. Shin DH, Feldman RM, Sheu WP, et al. Efficacy and safety of the fixed combinations latanoprost/timolol versus dorzolamide/timolol in patients with elevated intraocular pressure. Ophthalmology. 2004; 111:276–282.
13. Konstas AG, Lake S, Economou AI, et al. 24-Hour control with a latanoprost-timolol fixed combination vs timolol alone. Arch Ophthalmol. 2006; 124:1553–1557.
14. Martinez A, Sanchez M. Bimatoprost/timolol fixed combination vs latanoprost/timolol fixed combination in open-angle glaucoma patients. Eye (Lond). 2009; 23:810–818.
15. Lewis RA, Gross RL, Sall KN, et al. The safety and efficacy of bimatoprost/timolol fixed combination: a 1-year double-masked, randomized parallel comparison to its individual components in patients with glaucoma or ocular hypertension. J Glaucoma. 2010; 19:424–426.
16. Brandt JD, Cantor LB, Katz LJ, et al. Bimatoprost/timolol fixed combination: a 3-month double-masked, randomized parallel comparison to its individual components in patients with glaucoma or ocular hypertension. J Glaucoma. 2008; 17:211–216.
17. Konstas AG, Katsimbris JM, Lallos N, et al. Latanoprost 0.005% versus bimatoprost 0.03% in primary open-angle glaucoma patients. Ophthalmology. 2005; 112:262–266.
18. Robin AL, Covert D. Does adjunctive glaucoma therapy affect adherence to the initial primary therapy? Ophthalmology. 2005; 112:863–868.
19. Hasegawa K, Ishida K, Sawada A, et al. Diurnal variation of intraocular pressure in suspected normal-tension glaucoma. Jpn J Ophthalmol. 2006; 50:449–454.
20. Tajunisah I, Reddy SC, Fathilah J. Diurnal variation of intraocular pressure in suspected glaucoma patients and their outcome. Graefes Arch Clin Exp Ophthalmol. 2007; 245:1851–1857.
21. Sit AJ, Liu JH, Weinreb RN. Asymmetry of right versus left intraocular pressures over 24 hours in glaucoma patients. Ophthalmology. 2006; 113:425–430.
22. Liu JH, Sit AJ, Weinreb RN. Variation of 24-hour intraocular pressure in healthy individuals: right eye versus left eye. Ophthalmology. 2005; 112:1670–1675.
23. Lee JY, Hwang YH, Kim YY. The efficacy of a monocular drug trial in normal-tension glaucoma. Korean J Ophthalmol. 2012; 26:26–31.
24. Realini T, Fechtner RD, Atreides SP, Gollance S. The uniocular drug trial and second-eye response to glaucoma medications. Ophthalmology. 2004; 111:421–426.
25. Dayanir V, Cakmak H, Berkit I. The one-eye trial and fellow eye response to prostaglandin analogues. Clin Experiment Ophthalmol. 2008; 36:136–141.
26. Chaudhary O, Adelman RA, Shields MB. Predicting response to glaucoma therapy in one eye based on response in the fellow eye: the monocular trial. Arch Ophthalmol. 2008; 126:1216–1220.
27. Takahashi M, Higashide T, Sakurai M, Sugiyama K. Discrepancy of the intraocular pressure response between fellow eyes in one-eye trials versus bilateral treatment: verification with normal subjects. J Glaucoma. 2008; 17:169–174.
28. Noecker RS, Dirks MS, Choplin NT, et al. A six-month randomized clinical trial comparing the intraocular pressure-lowering efficacy of bimatoprost and latanoprost in patients with ocular hypertension or glaucoma. Am J Ophthalmol. 2003; 135:55–63.
29. Orzalesi N, Rossetti L, Bottoli A, Fogagnolo P. Comparison of the effects of latanoprost, travoprost, and bimatoprost on circadian intraocular pressure in patients with glaucoma or ocular hypertension. Ophthalmology. 2006; 113:239–246.
30. Parrish RK, Palmberg P, Sheu WP. XLT Study Group. A comparison of latanoprost, bimatoprost, and travoprost in patients with elevated intraocular pressure: a 12-week, randomized, masked-evaluator multicenter study. Am J Ophthalmol. 2003; 135:688–703.
31. Woodward DF, Krauss AH, Chen J, et al. Pharmacological characterization of a novel antiglaucoma agent, Bimatoprost (AGN 192024). J Pharmacol Exp Ther. 2003; 305:772–785.
32. Sharif NA, Kelly CR, Crider JY, et al. Ocular hypotensive FP prostaglandin (PG) analogs: PG receptor subtype binding affinities and selectivities, and agonist potencies at FP and other PG receptors in cultured cells. J Ocul Pharmacol Ther. 2003; 19:501–515.
33. Takagi Y, Nakajima T, Shimazaki A, et al. Pharmacological characteristics of AFP-168 (tafluprost), a new prostanoid FP receptor agonist, as an ocular hypotensive drug. Exp Eye Res. 2004; 78:767–776.
34. Cho SW, Kim JM, Park KH, Choi CY. Effects of brimonidine 0.2%-timolol 0.5% fixed-combination therapy for glaucoma. Jpn J Ophthalmol. 2010; 54:407–413.
35. Konstas AG, Boboridis K, Tzetzi D, et al. Twenty-four-hour control with latanoprost-timolol-fixed combination therapy vs latanoprost therapy. Arch Ophthalmol. 2005; 123:898–902.
36. Simsek S, Yulek F, Cakmak HB, Midillioglu IK. Long-term effects of prostaglandin analogues on the anterior chamber depth of patients with primary open-angle glaucoma. Cutan Ocul Toxicol. 2009; 28:125–128.
37. Gutierrez-Ortiz C, Teus MA, Bolivar G. Short-term effects of latanoprost on anterior chamber depth in patients with glaucoma or ocular hypertension. Invest Ophthalmol Vis Sci. 2006; 47:4856–4859.
38. Martinez A, Sanchez M. A comparison of the safety and intraocular pressure lowering of bimatoprost/timolol fixed combination versus latanoprost/timolol fixed combination in patients with open-angle glaucoma. Curr Med Res Opin. 2007; 23:1025–1032.
39. Chen J, Dinh T, Woodward DF, et al. Bimatoprost: mechanism of ocular surface hyperemia associated with topical therapy. Cardiovasc Drug Rev. 2005; 23:231–246.
40. Russo A, Riva I, Pizzolante T, et al. Latanoprost ophthalmic solution in the treatment of open angle glaucoma or raised intraocular pressure: a review. Clin Ophthalmol. 2008; 2:897–905.
41. Feldman RM. Conjunctival hyperemia and the use of topical prostaglandins in glaucoma and ocular hypertension. J Ocul Pharmacol Ther. 2003; 19:23–35.
42. Bhorade AM. The monocular trial controversy: a critical review. Curr Opin Ophthalmol. 2009; 20:104–109.
43. Drance SM. The uniocular therapeutic trial in the management of elevated intraocular pressure. Surv Ophthalmol. 1980; 25:203–205.
44. Piltz J, Gross R, Shin DH, et al. Contralateral effect of topical beta-adrenergic antagonists in initial one-eyed trials in the ocular hypertension treatment study. Am J Ophthalmol. 2000; 130:441–453.
45. Liu JH, Zhang X, Kripke DF, Weinreb RN. Twenty-four-hour intraocular pressure pattern associated with early glaucomatous changes. Invest Ophthalmol Vis Sci. 2003; 44:1586–1590.
46. Wax MB, Camras CB, Fiscella RG, et al. Emerging perspectives in glaucoma: optimizing 24-hour control of intraocular pressure. Am J Ophthalmol. 2002; 133:Suppl. S1–S10.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download