Abstract
Purpose
To determine effectiveness of laser-assisted in situ keratomileusis (LASIK) in the treatment of astigmatism following penetrating keratoplasty (PK).
Methods
We performed a retrospective review of medical records of patients who underwent LASIK following PK and had over 1 year of follow-up data.
Results
Twenty-six patients (26 pairs of eyes) underwent LASIK following PK. Mean age of the patients at the time of LASIK was 40.7 years (range, 26 to 72 years). Following LASIK, the mean cylinder was reduced by 2.4 diopters and mean reduction of cylinder after LASIK was 65.4% from the preoperative values at the last follow-up visit. Uncorrected visual acuity became 20 / 50 or better in 69.2% of the eyes after LASIK. Best-corrected visual acuity became 20 / 50 or better in 73.1% of the eyes after LASIK. All of them were intolerable to contact lenses before LASIK. After LASIK, 6 pairs (23.1%) did not need to use contact lenses and 18 pairs (69.2%) were tolerable to using contact lenses or spectacles. There were no significant endothelial cell density changes 12 months after LASIK (p = 0.239).
In the postkeratoplasty eye, astigmatism is the most frequent factor limiting visual acuity, binocular visual function, and patient satisfaction. A full spectacle correction of the postoperative refractive error is often poorly tolerated because of anisometropia. Contact lenses are effective in restoring good vision and binocularity in some but not all patients. Many patients are unable to tolerate or handle contact lenses. Photorefractive keratectomy (PRK) has been used after corneal transplantation. Some reports of PRK have been positive [1,2]. However, the results of PRK have been published in different studies, reporting a significant incidence of stromal haze, regression, and even severe scarring [3,4,5]. Advantages of laser-assisted in situ keratomileusis (LASIK) over PRK include the capacity to treat a greater range of refractive errors than PRK, rapid recovery of vision following surgery, a potential for enhancement, and a low risk of inducing corneal haze and scarring. We evaluated whether LASIK was an effective treatment for astigmatism after penetrating keratoplasty (PK) and increasing tolerance of contact lenses.
We reviewed the medical records of patients who underwent LASIK following PK and had over 1-year follow-up data available. The indication for LASIK was intolerance of spectacles and contact lenses. Preoperative evaluation included a complete eye examination, including uncorrected visual acuity (UCVA), best-corrected visual acuity (BCVA), manifest refraction, tonometry, corneal topography (Orbscan II; Bausch & Lomb, Rochester, New York, USA), and ultrasound pachymetry. Regular symmetric astigmatism was scheduled for LASIK. All sutures were removed at least 6 months prior to LASIK.
The surgery was performed under topical proparacaine anesthesia. The corneal flap was cut using the M2 microkeratome (Moria M2; Moria SA, Antony, France) and a 130- or 160-um flap with superior hinge was created. Photoablations were made using the VISK STAR S4 (VISX, Santa Clara, CA, USA) argon fluoride 193 nm eximer laser with a fluence of 160 mJ/cm2 using an optical zone of 6.0 mm. The LASIK flaps of all patients were consistently sized at 8.5 mm. The desired correction was emmetropia. The full refractive cylinder was treated. A nomogram based on experience in normal eyes was used in ablations of the spherical component.
Postoperatively, patients received 0.5% levofloxacin and 1% prednisolone acetate four times daily for the first operative two weeks. At each follow-up visit, UCVA and BCVA were measured. Slit-lamp examination and corneal topography using Orbscan II were performed.
The mean endothelial cell density was measured before LASIK and 12 months after the procedure. We used paired t-tests to compare the interval change of endothelial cell density. Statistical analyses were performed using SPSS ver. 18.0 (SPSS Inc., Chicago, IL, USA). Values with p < 0.05 were considered statistically significant. Tolerance of contact lenses was measured by estimating whether patients were able to wear contact lenses without decentering them and without consistent complaint of ocular discomforts after making every effort to adopt them during a 2-week period. All means are reported with their standard deviations and ranges.
Twenty-six pairs of eyes with astigmatism were treated. Twenty were male and six were female. Mean age was 40.7 ± 15.8 years (range, 26 to 72 years). Indications of PK were inflammatory corneal opacity (11 eyes), keratoconus (8 eyes), corneal macular dystrophy (3 eyes), bullous keratopathy (3 eyes), and iridocorneal endothelial syndrome (1 eye). Anisometropia with full spectacle correction and/or contact lens intolerance was the indication for LASIK following PK. No intraoperative complications occurred. Preoperative manifest refraction showed a mean spherical component of -0.64 ± 3.12 diopters (D; range, -4.25 to +3.75 D) and mean cylinder of -6.94 ± 2.40 D (range, -3.00 to -10.50 D). The interval from PK to LASIK surgery ranged from 11 to 178 months, with a mean of 47 months. Average duration of follow-up after LASIK was 25 months (range, 12 to 60 months).
Before LASIK, the UCVA was 20 / 200 or worse in 53.8% of eyes and all eyes had a UCVA worse than 20 / 60. At the last follow-up visit after LASIK, the UCVA became 20 / 50 or better in 18 pairs of eyes (69.2%) (Fig. 1). We gained a mean of 2.7 lines of UCVA and there was no loss of UCVA after LASIK (Fig. 2).
Before LASIK, 14 pairs of eyes (53.8%) had a BCVA of 20 / 50 or better. After LASIK, 19 pairs of eyes (73.1%) had a BCVA of 20 / 50 or better (Fig. 3). Four pairs of eyes (15.4%) had a loss of more than one line of BCVA because of increased corneal opacity (2 pairs) or irregular astigmatism (2 pairs). In seven pairs (26.8%), the BCVA remained unchanged from the pre-LASIK level. Fifteen pairs of eyes (57.7%) gained 1 line or more of BCVA (Fig. 4).
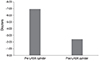
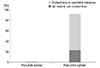
At the 12-month visit following LASIK, postoperative manifest refraction showed a mean spherical component of 0.98 ± 1.86 D (range, -2.25 to +3.25 D) and the mean cylinder was 2.40 ± 1.25 D (range, 0.25 to 4.25 D). Mean reduction in the cylinder was 4.54 D (65.4%) after the 12-month visit (Fig. 5). Before LASIK, none of the patients (0%) tolerated contact lenses or spectacles. However, after LASIK, six pairs of eyes (23.1%) did not need to use contact lenses and 18 pairs (69.2%) tolerated the use of contact lenses or spectacles (Fig. 6). Intolerance of contact lenses was diagnosed only in two pairs of eyes (7.7%).
Before LASIK, mean endothelial cell density in 26 pairs of eyes was 1,577.4 ± 457.2 cells/mm2 (range, 562.4 to 2,520.5 cells/mm2). After LASIK, mean endothelial cell density in 26 pairs of eyes was 1,509.3 ± 482.2 cells/mm2. There were no significant endothelial cell density changes 12 months after LASIK (p = 0.239).
To treat postkeratoplasty astigmatism, a first option is typically spectacles or contact lenses. However, spectacles have many limitations such as aniseikonia, the inability to correct significant high astigmatism or irregular astigmatism. Fitting contact lenses to postkeratoplasty patients may be challenging [6,7]. All postkertoplasty patients cannot easily adapt to contact lenses because of decentration and contact lens complications [8]. In our study, the mean reduction in cylinder was 4.54 D (65.4%) after LASIK. Furthermore, tolerance of contact lenses was 0% before LASIK. After LASIK, six pairs of eyes (23.1%) did not need to use contact lenses or spectacles and 18 pairs of eyes (69.2%) tolerated the use of contact lenses or spectacles. Therefore, LASIK after PK raised the rate of contact lens tolerance.
Contact lens intolerance was diagnosed only in two pairs of eyes (7.7%). The astigmatism of these two eyes was measured to be against the astigmatism rule of -4.25 D, which were -7.75, -10.5 D astigmatism before LASIK, and incompletely corrected by LASIK. Compared to the other eyes that tolerated the use of contact lenses, the only different finding of these intolerant eyes was a higher degree of residual astigmatism above -4.0 D, which was assumed to be a cause of intolerance.
In patients who did not tolerate spectacles and contact lenses, surgical options can be considered. Before we performed LASIK after PK, PRK was performed in four pairs of eyes. Two pairs of eyes had significant stromal haze and scarring. After LASIK, the other pairs of eyes (2 pairs) also had increased corneal opacity. However, the corneal opacity after PRK was more severe than that after LASIK.
We performed LASIK as a one-step procedure. LASIK requires the creation of a lamellar flap by microkeratome. The lamellar corneal flap can induce significant changes in the corneal shape, especially in PKP patients [9]. To raise the predictability of the LASIK outcome, a 2-step procedure (lamellar cut then ablation) may be better than one-step surgery. However, there was no difference in postoperative UCVA or BCVA, spherical equivalent, or cylinder [10].
Enhancement was made for residual cylinder in two pairs of eyes. Visual acuity after enhancement was included in postoperative data. In most recent studies of one-step LASIK, enhancement was observed in 9% to 39% of cases [11,12,13]. In our study, the rate of enhancement was 8%.
LASIK complications such as buttonholes, f lap dislocation, striae, and epithelial ingrowth were absent. Dehiscence of graft-host junction did not occur with high suction at the time of LASIK [14]. Graft rejection, failure, and corneal edema did not happen [15]. However, four pairs of eyes had a loss in Snellen lines of BCVA after LASIK because of increased corneal opacity (two pairs) and irregular astigmatism (two pairs). In particular, one of them even had a loss of three Snellen lines in BCVA because of severe corneal opacity, and in these cases, we performed rekeratoplasty. There was no endothelial cell loss after LASIK. In our study, corneal opacity and irregular astigmatism after LASIK were the major causes of decreasing BCVA.
We had over 2 years of follow-up data in only 10 pairs of eyes (38.5%). Refractive and keratometric stability was estimated f rom these interval changes of manifest refraction. At the 24-month visit following LASIK, manifest refraction showed a mean spherical component of -0.15 ± 1.75 D (range, -2.75 to +2.0 D) and the mean cylinder was 2.75 ± 1.25 D (range, 1.0 to 4.25 D).
In summary, LASIK is an effective treatment for reducing astigmatism following PK and increases contact lens and spectacle tolerance. However, we need much more knowledge about laser procedures and corneal wound healing after keratoplasty for better clinical outcomes.
Figures and Tables
References
1. Lazzaro DR, Haight DH, Belmont SC, et al. Excimer laser keratectomy for astigmatism occurring after penetrating keratoplasty. Ophthalmology. 1996; 103:458–464.
2. Bansal AK. Photoastigmatic refractive keratectomy for correction of astigmatism after keratoplasty. J Refract Surg. 1999; 15:2 Suppl. S243–S245.
3. Campos M, Hertzog L, Garbus J, et al. Photorefractive keratectomy for severe postkeratoplasty astigmatism. Am J Ophthalmol. 1992; 114:429–436.
4. Bilgihan K, Ozdek SC, Akata F, Hasanreisoglu B. Photorefractive keratectomy for post-penetrating keratoplasty myopia and astigmatism. J Cataract Refract Surg. 2000; 26:1590–1595.
5. Chan WK, Hunt KE, Glasgow BJ, Mondino BJ. Corneal scarring after photorefractive keratectomy in a penetrating keratoplasty. Am J Ophthalmol. 1996; 121:570–571.
6. Genvert GI, Cohen EJ, Arentsen JJ, Laibson PR. Fitting gas-permeable contact lenses after penetrating keratoplasty. Am J Ophthalmol. 1985; 99:511–514.
7. Koffler BH, Clements LD, Litteral GL, Smith VM. A new contact lens design for post-keratoplasty patients. CLAO J. 1994; 20:170–175.
8. Matsuda M, MacRae SM, Inaba M, Manabe R. The effect of hard contact lens wear on the keratoconic corneal endothelium after penetrating keratoplasty. Am J Ophthalmol. 1989; 107:246–251.
9. Kohnen T, Buhren J. Corneal first-surface aberration analysis of the biomechanical effects of astigmatic keratotomy and a microkeratome cut after penetrating keratoplasty. J Cataract Refract Surg. 2005; 31:185–189.
10. Alio JL, Javaloy J, Osman AA, et al. Laser in situ keratomileusis to correct post-keratoplasty astigmatism: 1-step versus 2-step procedure. J Cataract Refract Surg. 2004; 30:2303–2310.
11. Barraquer CC, Rodriguez-Barraquer T. Five-year results of laser in-situ keratomileusis (LASIK) after penetrating keratoplasty. Cornea. 2004; 23:243–248.
12. Buzard K, Febbraro JL, Fundingsland BR. Laser in situ keratomileusis for the correction of residual ametropia after penetrating keratoplasty. J Cataract Refract Surg. 2004; 30:1006–1013.
13. Hardten DR, Chittcharus A, Lindstrom RL. Long term analysis of LASIK for the correction of refractive errors after penetrating keratoplasty. Cornea. 2004; 23:479–489.
14. Ranchod TM, McLeod SD. Wound dehiscence in a patient with keratoconus after penetrating keratoplasty and LASIK. Arch Ophthalmol. 2004; 122:920–921.
15. Dawson DG, Hardten DR, Albert DM. Pocket of fluid in the lamellar interface after penetrating keratoplasty and laser in situ keratomileusis. Arch Ophthalmol. 2003; 121:894–896.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download