Abstract
Purpose
To investigate the effects of topical prostaglandin analogue drugs on the differentiation of adipocytes.
Methods
Differentiation of 3T3-L1 preadipocytes was induced with isobutylmethylxanthine, dexamethasone, and insulin. 3T3-L1 cells were exposed to 0.008, 0.08, 0.2 µM of latanoprost and travoprost. Reverse transcription polymerase chain reaction for mRNA expression of lipoprotein lipase and peroxisome proliferator-activated receptor γ 2 (PPARγ2), and glycerol-3-phosphate dehydrogenase (G3PDH) assays were performed to examine the effects on early and late differentiation, respectively. Also, glycerol assays were done to evaluate the effect of prostaglandin analogues on lipolysis after differentiation.
Results
Both prostaglandin analogues inhibited differentiation of preadipocytes. Topical prostaglandin analogues significantly decreased G3PDH activity, a marker of late differentiation. However, topical prostaglandin analogues did not change mRNA expressions of lipoprotein lipase and PPARγ2, markers of early differentiation. The activities of the early markers of differentiation were not changed significantly before and after growth arrest. Compared to latanoprost, travoprost decreased G3PDH activity more significantly (p < 0.05). Both prostaglandin analogues did not affect the lipolysis of differentiated adipocytes (p > 0.05).
Prostaglandin F2α (PGF2α) analogs such as latanoprost (Xalatan; Pharmacia, Peapack, NJ, USA) and travoprost (Travatan; Alcon, Fort Worth, TX, USA) are topical hypotensive drugs frequently used for treating ocular hypertension and glaucoma [1,2]. These prostaglandin (PG) drugs are well known for their efficacy and potency as well as for good patient compliance because they can be administered in a single daily dose. However, the use of these drugs may lead to adverse effects such as darkening of the periocular skin and iris, increased eyelash growth, cystoid macular edema, and conjunctival hyperemia [3]. Deepening of the upper lid sulcus and decreased dermatochalasis have been reported in topical PG users [4,5,6,7,8,9]. Recent clinical investigation suggests that fat atrophy is a possible mechanism for the development of a deep superior sulcus [10].
The 3T3-L1 preadipocyte cell line, originally obtained from mouse embryos, provides a useful model for characterizing the events responsible for adipocyte differentiation [11]. In culture, the cells acquire the morphological and biochemical characteristics of adipocytes upon treatment with a methylisobutylxanthine, dexamethasone, insulin (MDI) cocktail: MDI and fetal bovine serum (FBS) [12]. With induction, the cells undergo mitosis followed by growth arrest [13]. After growth arrest, the cells express adipose-specific genes, accumulate fat droplets, and mature into terminally-differentiated adipocytes [14]. The 3T3-L1 preadipocyte cell line was used to investigate the effects of PGs on adipose conversion in this study because of its established ability to mimic in vivo development.
PGs produce a wide variety of biological responses through their binding to plasma membrane receptors. Several classes of PG receptors have been identified and characterized. PGF2α exerts its effects through a specific interaction with FP receptors. The inhibitory effect of PGF2α on adipocyte differentiation has been shown by previous studies [15,16,17,18,19]. By combining with the cell surface FP receptor to activate mitogen-activated protein kinase, PGF2α has an antiadipogenic effect and inhibits a nuclear hormone receptor, peroxisome proliferator-activated receptor γ (PPARγ) [19]. As a result PGF2α blocks adipogenesis, the process by which orbital fibroblasts differentiate into adipocytes. A possible mechanism for the inhibition of 3T3-L1 adipocyte differentiation is through an FP receptor-mediated increase in intracellular calcium and DNA synthesis [20]. Thus, PGF2α acts as a negative modulator of adipose differentiation but the detailed effect of commercial topical PG drugs on adipocyte differentiation is still unclear. PG-induced fat atrophy could be caused by inhibiting early and/or late differentiation of preadipocytes, or by stimulating the lipolysis of differentiated adipocytes. The purpose of this study is to investigate the effects of topical PG drugs on the regulation of preadipocyte differentiation and/or on the lipolysis of differentiated adipocytes using the adipogenic cell line 3T3-L1.
Methylisobutylxanthine, dexamethasone, insulin, and free glycerol determination kit (free glycerol determination kit) were purchased from Sigma-Aldrich Chemical (St. Louis, MO, USA). A glycerol-3-phosphate dehydrogenase (G3PDH) activity assay kit was purchased from Takara Nio (GPDH activity assay kit; Shiga, Japan). FBS, Dulbecco's modified Eagle's medium, penicillin/streptomycin, and trypsin were purchased from GIBCO (Grand Island, NY, USA). Primers for mRNA were obtained from Genet Bio (Seoul, Korea). All other reagents and general lab chemicals were purchased from Sigma Sigma-Aldrich Chemical.
The effects of the PGF2α analogs, latanoprost (Xalatan; Pfizer, New York, NY, USA) and travoprost (Travatan, Alcon) in their commercial formulations, were investigated on adipose differentiation and intracellular, lipid storage. Each PG solution was diluted serially to 0.008, 0.08, 0.2, 0.4, 0.8 µM and was tested for cytotoxicity with MTT (3-[4, 5-dimethylthiazol-2-yl]-2, 5-diphenyltetrazolium bromide) assay. Solutions tested at a concentration of 0.4 µM appeared too toxic to be analyzed, even at 24 hours. However, compounds tested at a concentration of 0.2 µM did not reveal significant cytotoxicity.
3T3-L1 preadipocytes were maintained and differentiated as described [21]. The cultures were divided into three groups based on the stage of differentiation. On day 2 when the cells reached confluency, differentiation was induced by treating the cells for 48 hours with MDI cocktails consisting of 1 µg/mL dexamethasone, 0.5 mM isobutylmethylxanthine, and 10 µg/mL insulin and maintained for two days. During this time of growth arrest, PGs were added to the media to evaluate the effect of PGs before the start of differentiation. After the growth arrest, PGs were added to the media to evaluate their effect on the early or late stage of differentiation. In another group, the medium was replaced and PGs were added to the media on day 10 and maintained until day 14 to evaluate the effect on lipolysis after differentiation.
Total RNA was extracted with Trizol (Invitrogen, Carlsbad, CA, USA). Northern blot analysis to measure the mRNA expression of lipoprotein lipase (LPL) and PPARγ2 was performed before and after the growth arrest to evaluate the effects of PGs on early differentiation. Briefly, an RNA denaturation mix composed of isolated RNA, oligo dT primer, and nuclease-free water was used to denature the RNA. The polymerase chain reaction (PCR) primer pairs for cDNA amplification were as follows: LPL (forward) 5'-TGCCGCTGTTTTGTTTTACC-3' and (reverse) 5'-TCACAGTTTCTGCTCCCAGC-3', PPARγ2 (forward) 5'-TGCCGCTGTTTTGTTTTACC-3' and (reverse) 5'-AATCAGCAACCATTGGGTCA-3'. cDNA was synthesized by adding prime RT premix (Genet Bio). Taq Green Master Mix (Promega, Madison, WI, USA) and 10 pmol of forward primer and reverse primer were added to the synthesized cDNA, and amplified with annealing for 30 cycles with DNAEngine cycler (Bio-Rad, Hercules, CA, USA). The DNA band of amplified PCR products was analyzed by multi-Gauze (Fujifilm, Tokyo, Japan) after electrophoresis. The level of β-actin was used as an internal standard.
G3PDH, known as a late marker of differentiation, participates in the adipose conversion of 3T3 cells [22]. G3PDH activities were assessed on day 10 using commercial kits with the manufacturer's instructions. The absorbance was measured at 340 nm with a spectrophotometer (Fluostar Optima; BMG Labtech, Offenburg, Germany).
A glycerol assay was performed to evaluate the effects of PGs on the lipolysis of differentiated adipocytes on day 14. For the quantitative enzymatic determination of glycerol, a commercial free glycerol determination kit was used. The absorbance was measured at 540 nm with a spectrophotometer.
The expression of mRNA was scanned, and the relative intensity of the bands was determined by densitometry. Data are expressed as mean ± standard error of the mean corresponding to the number of wells analyzed. Experimental differences between control culture results and the single treatment groups were evaluated using Student's t-test with p-values less than 0.05 considered significant.
Adipose differentiation has been described as a cascade of events characterized at the molecular level by the induction of early and late markers of differentiation [23,24]. 3T3-L1 cells differentiated into adipocytes upon exposure to the MDI cocktail (Fig. 1).
Experiments were performed to determine whether topical PG analogues inhibited the expression of early markers of differentiation [25,26,27]. As a result, topical PG analogues did not increase the expression of mRNA for both LPL and PPARγ2 significantly (Fig. 2) (p > 0.05). The activities of early markers of differentiation were not changed significantly before and after growth arrest (Figs. 3 and 4).
The results shown in Fig. 5 indicate that the addition of topical PG analogues to the culture medium of 3T3-L1 cells inhibited the increase of G3PDH specific activity, a late marker of differentiation. The levels of G3PDH activity increased when 3T3-L1 started to differentiate after growth arrest. With exposure to latanoprost, there was a dose-dependent decrease in the G3PDH activity in the following order: 0.008 µM (92.82 ± 1.27%), 0.08 µM (60.21 ± 1.96%), and 0.2 µM (53.18 ± 1.47%). With exposure to travoprost, there was also a dose-dependent decrease in the G3PDH activity in the following order: 0.008 µM (67.97 ± 0.67%), 0.08 µM (53.07 ± 1.52%), and 0.2 µM (36.44 ± 0.97%). Both latanoprost and travoprost decreased G3PDH activity significantly (p < 0.05) compared to the non-exposed control, and travoprost decreased G3PDH activity more significantly compared to latanoprost at each concentration (p < 0.05).
These results showed that topical PG analogues inhibited the expression of differentiation markers when the cells had already started to differentiate, especially at the late stage of differentiation.
Since decreased adipose tissue can be caused by increased lipolysis, an experiment was performed to determine the stimulating effect of topical PG analogues on the lipolysis of adipocytes. On day 10, when differentiation had already started, PGs were added and maintained, and a glycerol assay was performed on day 14. Glycerol is a better marker of lipolytic rates, because unlike fatty acids, glycerol is not oxidized or reesterified by adipocytes. As shown in Fig. 6, the addition of topical PG analogues to the differentiated adipocytes did not increase glycerol accumulation in the medium, indicating that topical PG analogues did not significantly stimulate lipolysis compared to the non-exposed control (p > 0.05).
The results presented in this study demonstrate that topical PGF2α agonists are inhibitors of adipose differentiation in 3T3-L1 cells.
The 3T3-L1 cell line is one of the most well-characterized and reliable models for studying the conversion of preadipocytes into adipocytes [28]. In culture, differentiated 3T3-L1 preadipocytes possess most of the ultrastructural characteristics of adipocytes from animal tissue. The formation and appearance of developing fat droplets also mimic live adipose tissue. Confluent 3T3-L1 preadipocytes can be differentiated synchronously by a defined adipogenic cocktail. After induction, differentiating preadipocytes undergo a post-confluent mitosis and subsequent growth arrest. After growth arrest, cells are committed to becoming adipocytes. Adipose differentiation corresponds to a sequential series of events characterized by the induction of early markers of differentiation, followed by the induction of late markers of differentiation.
The prostanoids are an established group of ocular hypotensive drugs used for the clinical management of glaucoma. Topical application of the prodrug latanoprost (ester) leads to absorption of approximately 1% of the drug through the cornea, where it is completely hydrolyzed to the biologically-active acid form. The PG transporter OATP2A1 is expressed in human ocular tissues and transports the antiglaucoma prostanoids [29]. These compounds have been proven to be highly efficient agents for lowering intraocular pressure, with few local and systemic side effects.
The order of potency of various PGs suggests that inhibition of differentiation may be mediated by binding to the PGF2α receptor, which is classified as an FP receptor [30]. A previous study reported a possible mechanism for PGF2α in acting as a barrier to the 3T3-L1 differentiation program through a specific FP receptor. Membranes prepared from both 3T3-L1 preadipocytes and adipocytes exhibited a specific binding for PGF2α [20]. In contrast, compounds structurally related to PGE2 such as 17-phenyltrinor PGE2, had no effect on adipose differentiation except when added at a 10,000-fold higher concentration [18]. Activation of the FP receptor resulted in a transient increase in intracellular calcium, which is known to occur through the G protein-mediated activation of phospholipase C. Reginato et al. [19] showed that PGF2α has an antiadipogenic effect by combining with the cell surface FP receptor to activate mitogen-activated protein kinase, and that it inhibits the nuclear hormone receptor, PPARγ. FP receptors are present at similar levels in both preadipocytes and adipocytes, and the FP receptor-mediated inhibition of differentiation appears to be regulated by alterations in the PGF2α concentration, rather than in receptor expression [20]. Since PGF2α inhibits the differentiation of newborn rat adipocyte precursors in primary culture in defined medium [31], the inhibitory effect on the adipose differentiation observed with PGF2α is likely physiological. The reduction of PGF2α may allow cells to progress into terminal differentiation as PGF2α concentrations decrease during 3T3-L1 differentiation. Thus when PG drops stopped, the antiadipogenic effect of PG could be reversible.
Expression of mRNA for LPL and PPAR2γ, early markers of adipocyte differentiation, was examined in cells treated with PG analogues in this study. Expression of mRNA for both early markers was not changed in the presence of latanoprost or travoprost.
Both latanoprost and travoprost inhibit G3PDH specific activity in a dose dependent manner. With exposure to travoprost, G3PDH activity was more significantly decreased compared to latanoprost in this study. The discrepancy in the inhibitory effects between latanoprost and travoprost on the differentiation of adipocytes may be explained by the level of affinity to the FP receptors since travoprost is known to have a higher FP receptor affinity than latanoprost. Accumulation of glycerol was not inhibited when differentiated adipocytes were exposed to latanoprost or travoprost. These findings suggest that latanoprost and travoprost do not stimulate lipolysis of differentiated adipocytes.
Since Peplinski and Albiani Smith [4] first reported the upper eyelid sulcus deepening and dermatochalasis involution in three Caucasian patients treated with bimatoprost unilaterally, other similar clinical observations have been reported [5,6,7,8,9,10]. Previous studies suggest that the common pathophysiologic mechanism of periocular changes by PG analogues is atrophy of preaponeurotic and deep orbital fat [6,7]. A recent histological study also suggests orbital fat atrophy as a mechanism of upper eyelid sulcus deepening in topical PG analog users, including those using bimatoprost [10]. PGF2α analogs exhibit different agonist activities in experimental and clinical applications; this has been attributed to structural differences between the PGF2α analogs, differences in FP receptor affinity, or minor effects on other PG receptors [32,33]. A study with human orbital preadipocytes showed that latanoprost had the weakest antiadipogenic effect, and bimatoprost induced the most significant reduction of adipogenesis [34]. As inhibition of adipose differentiation by PGF2α can be reversible [35], the inhibitory effects of topical PGs may be important for the short term regulation of adipocyte function in vivo within the adipose tissue. Future in vivo experiments are necessary to investigate this possibility and determine the physiological significance of this antiadipogenic process of PGF2α in adipose tissue.
This study demonstrates that there is a greater suppression of preadipocyte differentiation rather than lipolysis in adipocytes. Another study has demonstrated that PGs themselves directly stimulate lipolysis in cultured 3T3 adipocytes [36]. This discrepancy may result from a cytokine-induced increase in PG synthesis, which would be expected to increase lipolysis since orbital fat tissue represents a highly specialized adipose tissue depot that occupies the space behind the eyeball [37]. Orbital fat tissue is biologically distinct from the omental and subcutaneous fat tissue, not only in morphological phenotypic features, but also at a molecular level with respect to expression of distinct surface receptors, cellular proteins, and responses to cytokines or hormones [38,39]. Inhibition of preadipocyte differentiation or reduction of fat accumulation in adipocytes caused by hormones, drugs, or PGs might be linked to orbital volume reduction, and volume deficits of the orbit may lead to deep superior sulcus syndrome and enophthalmos in vivo.
In summary, PGF2α inhibits the expression of markers that are induced when the cells have just starting to differentiate. The addition of PGFα when the cells had just started to differentiate was sufficient to inhibit 3T3-L1 cell differentiation as PGF2α was added during the differentiation period, and the expression of mRNA for the late marker of differentiation (G3PDH activity) was decreased. In contrast, PGF2α added before the onset of the differentiation program had a much less inhibitory effect on differentiation. The results presented here also indicate that PGF2α is less effective in blocking differentiation once the cells have started to differentiate. To conclude, topical antiglaucoma PG drugs inhibited the differentiation of preadipocytes especially at the late stage of differentiation. Although reversible in some cases after cessation, this inhibitory effect of PG on orbital adipose precursors and adipogenesis could be an important pathophysiologic mechanism of upper eyelid sulcus deepening in topical PG analog users. Thus, when prescribing these drugs, this adverse effect should be explained to patients and monitoring for possible adverse effects should be performed.
Figures and Tables
Fig. 1
Photomicrograph of 3T3-L1 cells cultivated in the presence (A) or absence of MDI (methylisobutylxanthine, dexamethasone, insulin) cocktail (B). Differentiation was initiated by treating the cells for 48 hours with 1 mg/mL dexamethasone, 0.5 mM isobutylmethylxanthine, and 10 mg/mL insulin (×100).

Fig. 2
Effect of topical prostaglandin (PG) analogues on the activity of mRNA expression of lipoprotein lipase (A) and peroxisome proliferator-activated receptor γ 2 (PPARγ2, early differentiation markers) (B) in 3T3-L1 preadipocytes. 3T3-L1 cells were cultivated in defined medium alone (control, C) or in the presence of 0.2, 0.08, 0.008 µM latanoprost (L) or 0.2, 0.08, 0.008 µM travoprost (T). Topical PGs did not increase the expression of mRNA of lipoprotein lipase or PPARγ2.

Fig. 3
Effect of topical prostaglandin (PG) analogues on the expression of mRNA of lipoprotein lipase in 3T3-L1 preadipocytes before and after growth arrest. Neither PG analogue significantly changed the activity of lipoprotein lipase before and after growth arrest (p > 0.05).
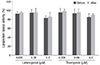
Fig. 4
Effect of topical prostaglandin (PG) analogues on the expression of mRNA of peroxisome proliferator-activated receptor γ 2 (PPARγ2) in 3T3-L1 preadipocytes before and after growth arrest. Neither PG analogue significantly changed the activity of PPARγ2 before and after growth arrest (p > 0.05).

Fig. 5
Effects of topical prostaglandin (PG) analogues on the activity of glycerol-3-phosphate dehydrogenase (G3PDH, late differentiation marker) in 3T3-L1 preadipocytes. 3T3-L1 cells were cultivated in defined medium, as described in Materials and Methods section. PG analogues were added when differentiation started on day 4, and their application was maintained throughout the experiment. Cells were harvested on day 10 to measure G3PDH specific activity (%). Both latanoprost and travoprost decreased G3PDH activity significantly in a dose-dependent manner compared to the non-exposed control (*p < 0.05). Travoprost decreased G3PDH activities more than latanoprost at each concentration (**p < 0.05).

Fig. 6
Effects of topical prostaglandin (PG) analogues on the stimulation of 3T3-L1 cell lipolysis with glycerol assay. 3T3-L1 cells were cultivated in defined medium, as described in Materials and Methods section. PG analogues were added after differentiation and maintained throughout the experiment. Cells were harvested on day 14 to measure glycerol (µg/mL). Addition of latanoprost or travoprost to the differentiated adipocytes did not increase glycerol accumulation in the medium compared to the non-exposed control (p > 0.05).
References
1. Krauss AH, Woodward DF. Update on the mechanism of action of bimatoprost: a review and discussion of new evidence. Surv Ophthalmol. 2004; 49:Suppl 1. S5–S11.
2. Hylton C, Robin AL. Update on prostaglandin analogs. Curr Opin Ophthalmol. 2003; 14:65–69.
3. Vogel R, Strahlman E, Rittenhouse KD. Adverse events associated with commonly used glaucoma drugs. Int Ophthalmol Clin. 1999; 39:107–124.
4. Peplinski LS, Albiani Smith K. Deepening of lid sulcus from topical bimatoprost therapy. Optom Vis Sci. 2004; 81:574–577.
5. Lee JW, Kim DY, Lee YK. Two cases of deepening of the upper lid sulcus from topical bimatoprost therapy. J Korean Ophthalmol Soc. 2007; 48:332–336.
6. Filippopoulos T, Paula JS, Torun N, et al. Periorbital changes associated with topical bimatoprost. Ophthal Plast Reconstr Surg. 2008; 24:302–307.
7. Tappeiner C, Perren B, Iliev ME, et al. Orbital fat atrophy in glaucoma patients treated with topical bimatoprost: can bimatoprost cause enophthalmos? Klin Monbl Augenheilkd. 2008; 225:443–445.
8. Yam JC, Yuen NS, Chan CW. Bilateral deepening of upper lid sulcus from topical bimatoprost therapy. J Ocul Pharmacol Ther. 2009; 25:471–472.
9. Yang HK, Park KH, Kim TW, Kim DM. Deepening of eyelid superior sulcus during topical travoprost treatment. Jpn J Ophthalmol. 2009; 53:176–179.
10. Park J, Cho HK, Moon JI. Changes to upper eyelid orbital fat from use of topical bimatoprost, travoprost, and latanoprost. Jpn J Ophthalmol. 2011; 55:22–27.
11. Green H, Kehinde O. Sublines of mouse 3T3 cells that accumulate lipid. Cell. 1974; 1:113–116.
12. Student AK, Hsu RY, Lane MD. Induction of fatty acid synthetase synthesis in differentiating 3T3-L1 preadipocytes. J Biol Chem. 1980; 255:4745–4750.
13. Cornelius P, MacDougald OA, Lane MD. Regulation of adipocyte development. Annu Rev Nutr. 1994; 14:99–129.
14. Green H, Meuth M. An established pre-adipose cell line and its differentiation in culture. Cell. 1974; 3:127–133.
15. Casimir DA, Miller CW, Ntambi JM. Preadipocyte differentiation blocked by prostaglandin stimulation of prostanoid FP2 receptor in murine 3T3-L1 cells. Differentiation. 1996; 60:203–210.
16. Abramovitz M, Boie Y, Nguyen T, et al. Cloning and expression of a cDNA for the human prostanoid FP receptor. J Biol Chem. 1994; 269:2632–2636.
17. Serrero G, Lepak N. Endocrine and paracrine negative regulators of adipose differentiation. Int J Obes Relat Metab Disord. 1996; 20:Suppl 3. S58–S64.
18. Serrero G, Lepak NM. Prostaglandin F2alpha receptor (FP receptor) agonists are potent adipose differentiation inhibitors for primary culture of adipocyte precursors in defined medium. Biochem Biophys Res Commun. 1997; 233:200–202.
19. Reginato MJ, Krakow SL, Bailey ST, Lazar MA. Prostaglandins promote and block adipogenesis through opposing effects on peroxisome proliferator-activated receptor gamma. J Biol Chem. 1998; 273:1855–1858.
20. Miller CW, Casimir DA, Ntambi JM. The mechanism of inhibition of 3T3-L1 preadipocyte differentiation by prostaglandin F2alpha. Endocrinology. 1996; 137:5641–5650.
21. Bernlohr DA, Bolanowski MA, Kelly TJ Jr, Lane MD. Evidence for an increase in transcription of specific mRNAs during differentiation of 3T3-L1 preadipocytes. J Biol Chem. 1985; 260:5563–5567.
22. Wise LS, Green H. Participation of one isozyme of cytosolic glycerophosphate dehydrogenase in the adipose conversion of 3T3 cells. J Biol Chem. 1979; 254:273–275.
23. Green H. Adipose conversion: a program of differentiation. In : Ailhaud G, editor. Obesity: cellular and molecular aspects. Paris: Colloques INSERM;1979. p. 15–24.
24. Amri EZ, Dani C, Doglio A, et al. Coupling of growth arrest and expression of early markers during adipose conversion of preadipocyte cell lines. Biochem Biophys Res Commun. 1986; 137:903–910.
25. Bernlohr DA, Angus CW, Lane MD, et al. Expression of specific mRNAs during adipose differentiation: identification of an mRNA encoding a homologue of myelin P2 protein. Proc Natl Acad Sci U S A. 1984; 81:5468–5472.
26. Tontonoz P, Hu E, Graves RA, et al. mPPAR gamma 2: tissue-specific regulator of an adipocyte enhancer. Genes Dev. 1994; 8:1224–1234.
27. Tontonoz P, Hu E, Spiegelman BM. Stimulation of adipogenesis in fibroblasts by PPAR gamma 2, a lipid-activated transcription factor. Cell. 1994; 79:1147–1156.
28. Ntambi JM, Kim YC. Adipocyte differentiation and gene expression. J Nutr. 2000; 130:3122S–3126S.
29. Kraft ME, Glaeser H, Mandery K, et al. The prostaglandin transporter OATP2A1 is expressed in human ocular tissues and transports the antiglaucoma prostanoid latanoprost. Invest Ophthalmol Vis Sci. 2010; 51:2504–2511.
30. Dukes M, Russell W, Walpole AL. Potent luteolytic agents related to prostaglandin F2alpha. Nature. 1974; 250:330–331.
31. Serrero G, Lepak NM, Goodrich SP. Prostaglandin F2 alpha inhibits the differentiation of adipocyte precursors in primary culture. Biochem Biophys Res Commun. 1992; 183:438–442.
32. Sharif NA, Kelly CR, Crider JY, et al. Ocular hypotensive FP prostaglandin (PG) analogs: PG receptor subtype binding affinities and selectivities, and agonist potencies at FP and other PG receptors in cultured cells. J Ocul Pharmacol Ther. 2003; 19:501–515.
33. Sharif NA, Crider JY, Husain S, et al. Human ciliary muscle cell responses to FP-class prostaglandin analogs: phosphoinositide hydrolysis, intracellular Ca2+ mobilization and MAP kinase activation. J Ocul Pharmacol Ther. 2003; 19:437–455.
34. Choi HY, Lee JE, Lee JW, et al. In vitro study of antiadipogenic profile of latanoprost, travoprost, bimatoprost, and tafluprost in human orbital preadiopocytes. J Ocul Pharmacol Ther. 2012; 28:146–152.
35. Lepak NM, Serrero G. Prostaglandin F2 alpha stimulates transforming growth factor-alpha expression in adipocyte precursors. Endocrinology. 1995; 136:3222–3229.
36. Chernick SS, Spooner PM, Garrison MM, Scow RO. Effect of epinephrine and other lipolytic agents on intracellular lipolysis and lipoprotein lipase activity in 3T3-L1 adipocytes. J Lipid Res. 1986; 27:286–294.
37. Feingold KR, Doerrler W, Dinarello CA, et al. Stimulation of lipolysis in cultured fat cells by tumor necrosis factor, interleukin-1, and the interferons is blocked by inhibition of prostaglandin synthesis. Endocrinology. 1992; 130:10–16.
38. Wolfram-Gabel R, Kahn JL. Adipose body of the orbit. Clin Anat. 2002; 15:186–192.
39. Bujalska IJ, Durrani OM, Abbott J, et al. Characterisation of 11beta-hydroxysteroid dehydrogenase 1 in human orbital adipose tissue: a comparison with subcutaneous and omental fat. J Endocrinol. 2007; 192:279–288.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download