Abstract
Purpose
Methods
Results
Figures and Tables
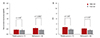 | Fig. 1Changes in visual acuities and ocular involvement score (OIS) with respect to patient age. (A) Mean logarithms of the minimal angle of resolution (logMAR) values in the pediatric group were similar at initial and final visits. In contrast, the mean logMAR of the adult patients improved significantly over the same period. (B) The pediatric group also showed no significant difference in mean OIS between initial and final visits. However, the adult group showed a significant improvement in mean OIS. There was no significant between group differences in each visits. Pediatric group = who are aged 18 years or less; adult group = who are over 18 years old. *Wilcoxon's signed rank test. |
 | Fig. 2Changes in visual acuities of the adult group with respect to treatment modality and time of treatment initiation. Early treatment of adult patients with intravenous immunoglobulin (IVIG) or with systemic corticosteroids was found to be associated with a significantly improved logMAR at final visit. The mean logMAR of patients treated with amniotic membrane graft transplantation (AMT) improved significantly by the final visit if patients were treated 15 days after disease onset or if their ocular involvement score was less than 6 at the initial visit. There was no significant between group differences in each visits. Early group = patients with treatment initiation ≤6 days for IVIG, ≤5 days for corticosteroid, or ≤15 days for AMT. Late group = treatment initiation >6 days for IVIG, >5 days for corticosteroid, or >15 days for AMT. Better group = patients with ocular involvement scores (OIS) of less than 6 at initial visit. Worse group = patients with OIS over 6 at initial visit. *Wilcoxon's signed rank test. |
 | Fig. 3Changes in ocular involvement score (OIS) of the adult group with respect to treatment modality and time of treatment initiation. Early treatment of adult patients with intravenous immunoglobulin (IVIG) or with systemic corticosteroids was found to be associated with a significantly improved OIS at final visit. The mean OIS of patients who were treated with amniotic membrane graft transplantation (AMT) improved significantly in the late group and worse group. There was no significant between group differences in each visits. Early group = patients with treatment initiation ≤6 days for IVIG, ≤5 days for corticosteroid, or ≤15 days for AMT. Late group = treatment initiation >6 days for IVIG, >5 days for corticosteroid, or >15 days for AMT. Better group = patients with OIS of less than 6 at initial visit. Worse group = patients with OIS over 6 at initial visit. *Wilcoxon's signed rank test. |
Table 2

Values are presented as number of patients (%) unless otherwise indicated.
IVIG = intravenous immunoglobulin; AMT = amniotic membrane graft transplantation.
*Supportive care includes adequate control of environmental temperature at 30℃ to 32℃, proper fluid balance management and wound care. And all other treatment modalities were accompanied by supportive care; †Data are presented as hydrocortisone equivalent dose.
Table 3
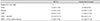
Data are presented as odds ratio (95% confidence intervals) for improvements in visual acuity and ocular involvement. Logistic regression analysis was used to calculate odds ratios.
BCVA = best-corrected visual acuity; IVIG = intravenous immunoglobulin; AMT = amniotic membrane graft transplantation.
Notes
Appendix
Appendix 1
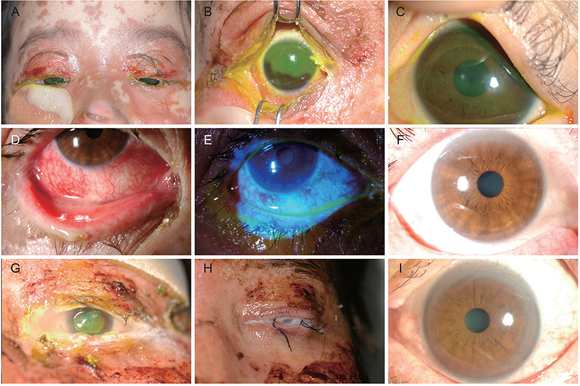
Clinical outcomes of representative cases enrolled in this study. (A-C) Gross and slitlamp photographs of 7-year-old girl with Stevens-Johnson syndrome at initial visit (A,B) and last visit (C). (D-F) Slitlamp photograph of a 59-year-old woman with toxic epidermal necrolysis at initial visit (D,E) and at last visit (F). (G-I) 31-Year-old man with Stevens-Johnson syndrome at initial visit (G) underwent amniotic membrane graft transplantation (H), which resulted in clinical improvement at last visit (I).




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download