Abstract
Purpose
Methods
Results
Figures and Tables
 | Fig. 1The change in (A) spherical equivalent, (B) astigmatism, (C) 3-mm zone irregular astigmatism, and (D) 5-mm zone irregular astigmatism at 1-year and final follow-up after deep anterior lamellar keratoplasty (DALK) and penetrating keratoplasty (PKP) with same-size donor graft. (A) Postoperative spherical equivalent refractive error was not different between DALK and PKP groups. (B) The incidence of astigmatism was not different between DALK and PKP groups for all follow-up periods. (C) Corneal irregularity indices measuring 3 mm were similar in DALK and PKP groups at all follow-up periods. (D) 5-mm corneal irregularity indices were less in the DALK group than in the PKP group at 1-year follow-up; however, there was only marginal significance at final follow-up (p = 0.084). Solid arrow: mean time of final suture removal in the PKP group. *Statistically significant (p < 0.05, Mann-Whitney test). (*) Marginal significance (0.05 < p < 0.10, Mann-Whitney test). |
 | Fig. 2(A) Illustration of the equilibrium of forces acting on the graft. Tensile forces of the peripheral recipient cornea act downward and counteract the 2 upward forces provided by the pressure of the aqueous humor and the pushing force of the recipient bed against the graft. (B) Illustration of a flattened recipient bed when the stiffness of the residual recipient bed decreases (for example, in a thinner residual bed). The graft exerts effective compressive force on the recipient bed, which flattens easily, resulting in flattening of the graft. (C) Illustration of a steepened graft when the stiffness of the residual recipient bed increases (for example, in a thicker residual bed) and stiffness of the peripheral corneal decreases (for example, in the thinner peripheral cornea seen in advanced keratoconus cases). The residual cornea would resist the flattening force exerting downward pressure, resulting in steepening of the graft. |
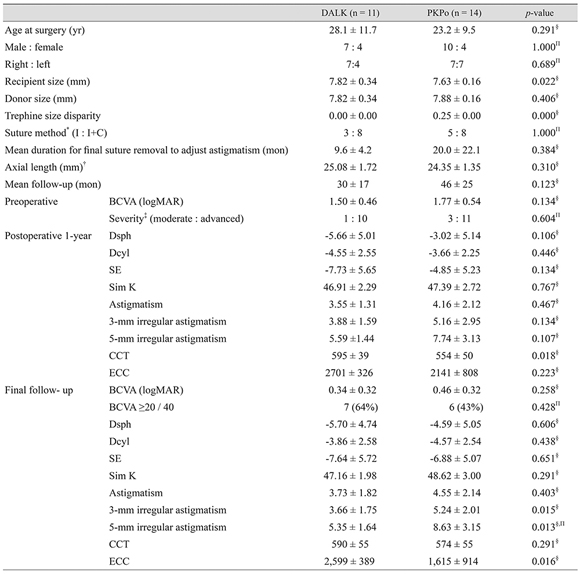
Table 1

DALK = deep anterior lamellar keratoplasty; PKP = penetrating keratoplasty; BCVA = best-corrected visual acuity; logMAR = logarithm of the minimal angle of resolution.
*Moderate (mean keratometric value <55 diopter [D]) : advanced keratoconus (mean keratometric value ≥55 D or unmeasurable keratometric value); †Interrupted suture only (I) : combined interrupted suture with continuous suture (I+C); ‡Preoperative axial length was not measured for every patient; §Mann-Whitney test; ΠFisher's exact test.
Table 3

Table 4

Trephine = trephine discrepancy; SE = spherical equivalent; D = diopter; Cyl = average cylinder; AXL = axial length; Preop K = preoperative keratometric value; Postop K = postoperative keratometric value; CT = corneal thickness; FU = follow-up duration; B = big-bubble technique; NA = not applicable; M = Melles technique.
*Median value; †Mean keratometric value in 3.0-mm zone; ‡Median value of recipient size and donor graft size were 8.0 mm, respectively; §Maximum keratometry, not simulated keratometry; ΠKeratoconus and other disease; #Hydrodissection technique using balanced salt solution; **Three cases of failed keratometry were excluded.
Appendix
Appendix 1
Comparison between DALK with same-size grafting and PKP with 0.25-mm oversize grafting




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download