Abstract
To report the surgical outcome of full-thickness sclerotomy in five cases of uveal effusion syndrome (UES). Full-thickness sclerotomy without sclerectomy was performed on five eyes of four patients with UES with or without nanophthalmos. In four of the eyes, exudative retinal detachment associated with UES resolved after the sclerotomy. The subretinal fluid in one eye, which had a normal axial length, was relieved after undergoing three sclerotomy procedures. Full-thickness sclerotomy without vortex vein decompression or sclerectomy is an effective surgical option for the management of significant UES.
After von Graefe [1], Verhoeff and Waite [2] defined spontaneous serous detachment of the choroid in 1858, uveal effusion syndrome (UES) was reported by Schepens and Brockhurst [3] and described as a nanophthalmic disorder with a scleral abnormality [4]. It was characterized by the accumulation of fluid escaped from the choriocapillaries into nearby free space, resulting from compressed vortex veins due to a thickened sclera. This would result in serous retinal and choroidal detachment and a thick choroid. Gass [5] and Gass and Jallow [6] described idiopathic UES and hypothesized that the cause was a congenital anomaly of the sclera and vortex veins that was related to aging, hormonal changes, or impairment of the permeability of the sclera. With the development of laboratory modalities, leopard-spot pattern changes on fluorescence angiography (FAG) and diffuse hyperfluorescence in the choroid on indocyanine green angiography (ICGA) were established as angiographic findings of UES [5-7]. Using histological methods, Trelstad et al. [8] reported the scleral abnormalities of UES. Forrester et al. [9] observed proliferation and migration of retinal pigment epithelial cells into the subretinal space. Synthesizing these findings, leopard-spot changes were believed to be the foci of retinal pigment epithelial proliferations and multi-layering.
For the treatment of UES, Brockhurst [10] described good surgical results with decompression of the vortex veins by scleral resection with sclerotomy. Gass [5] and Grass [11] reported an effective surgical procedure for sclerectomy and sclerostomy without decompression of the vortex veins due to the difficulty of isolating the vortex veins. Subsequent articles have provided evidence of the pertinence of scleral resection and sclerostomy [12-15]. Uyama et al. [7] treated 19 eyes of 16 patients with UES by making a two-thirds thickness scleral flap and performing a scleral excision to expose the underlying choroid. In our study, we reported the surgical outcome of drainage sclerotomy without vortex vein decompression or sclerectomy for the management of five cases of UES developed in Korean subjects.
After a radial conjunctival incision, the bare sclera was exposed. A full-thickness scleral incision (5 to 6 mm wide, 2 to 3 mm long, and square bracket shaped) was made in the quadrant between the rectus muscles. The anterior margin of the incision was usually 6 to 8 mm from the corneoscleral limbus. A small amount of supra-choroidal fluid was spontaneously drained after the sclerotomy. The underlying choroid was then exposed by meticulous dissection. The full-thickness scleral flap was then loosely sutured with 8-0 vicryl at two corners of the rectangle for a loose approximation at its original position (Fig. 1).
A 43-year-old man was referred to our retinal clinic due to decreased visual acuity in the left eye lasting for one month. He had been wearing glasses for hyperopia since he was younger than ten years. A gradual decrease in visual acuity led him to seek care at another hospital, where he was found to have a serous retinal detachment in the left eye. He was subsequently referred to our clinic.
At presentation, his best-corrected visual acuity (BCVA) was 0.3 and 0.01 logarithm of minimum angle resolution (logMAR), and spherical equivalents were +14.0 diopters (D) and +16.0 D in the right and left eyes, respectively. Fundus examination revealed nanophthalmic fundi in both eyes. In the left eye, there was uveal effusion involving the macula. The axial length was 16.41 mm in the right eye and 16.24 mm in the left eye on A-scan and B-scan ultrasonograms. Optical coherence tomography (OCT) showed a thickened sclera and retinal detachment (Fig. 2A). Under general anesthesia, a drainage sclerotomy was conducted in all four quadrants of the left eye. One month postoperatively, a flat retina was seen on fundus examination and OCT (Fig. 2B). The retina remained attached during the seven-month follow-up period, and an improved BCVA of 0.3 logMAR was achieved. We did not observe subretinal fluid or choroidal effusion in the fellow eye.
This case involved a 46-year-old man with blurry vision in the left eye for one week. For 15 years, he had receiving treatment with an intraocular pressure lowering medication at another hospital, and he was treated at our hospital with trabeculectomy one month prior to this second presentation under the diagnosis of congenital glaucoma. The refractive error was +3.5 D bilaterally, and BCVA was 0.4 logMAR before his experience with blurred vision. The anterior segment was normal with a proper filtering bleb, but examination of the posterior segment showed a total retinal and choroidal detachment (Fig. 2C). Retinal detachment with choroidal effusion was confirmed on ultrasound examination. The scleral thickness and axial length in the left eye measured by ultrasound were 1.94 and 22.94 mm, respectively. Treatment with oral steroids (50 mg oral prednisolone per day) was ineffective. We performed three rounds of drainage sclerotomy. Sclerotomy was performed at the superonasal and inferonasal quadrants during the first surgery. An abnormally rigid and thick sclera was observed during surgery. The first surgery resulted in recurrence of uveal effusion three days postoperatively. During the second surgery, we added two drainage sclerotomies in the same quadrants as those of the first surgery. UES recurred eight weeks postoperatively, and then a third surgery was performed in the inferotemporal quadrant. After the final surgery, the subretinal fluid gradually resolved at one month postoperatively (Fig. 2D). The final BCVA measured one month after the last surgery was 0.02 logMAR.
A 77-year-old man presented to the emergency room with a ten-day history of a shadow that affected the upper part of the vision in his left eye. He had no relevant ocular history, but had undergone cataract surgery in both eyes four years prior to presentation. BCVA without any refractive error was 1.0 and 0.4 logMAR in the right and left eyes, respectively. Fundus examination revealed marked shifting of the subretinal fluid underneath the retinal detachment of the left eye. On OCT examination, the retinal findings looked like an atypical central serous chorioretinopathy (Fig. 2E). For further workup, ultrasonography, FAG, and ICGA were performed. The axial length was 21.74 mm in the right eye and 21.56 mm in the left eye, which was not compatible with nanophthalmos. There were no definite leaking points on ICG, but leopard-spot patterns were noted on FAG. On the basis of these findings, we diagnosed him with UES. Despite treatment with steroids (50 mg oral prednisolone per day), the retinal findings and vision did not improve. Drainage sclerotomy was conducted in the inferotemporal and inferonasal quadrants. External subretinal fluid drainage was also performed. Ten days later, only a small amount of subretinal fluid was noted on fundus examination. His visual acuity improved to 0.6 logMAR three months after surgery, and no subretinal fluid was noted on OCT (Fig. 2F). His visual acuity improved to 0.9 logMAR, and the retina was stable at the last visit seven months postoperatively.
A 63-year-old man presented with impaired visual acuity in both eyes since his youth. He had no specific ocular or medical history, except for a ten-year history of diabetes mellitus. His BCVA was 0.03 logMAR in the right eye and 0.01 logMAR in the left eye with +13.0 D bilaterally. The intraocular pressure was found to be 14 mmHg in the right eye and 11 mmHg in the left eye using a Goldmann applanation tonometer. Slit lamp biomicroscopy examination showed anterior chambers as deep as four times the central corneal thickness in both eyes, and there was moderate nuclear sclerosis in the crystalline lens. Fundus examination demonstrated no diabetic retinopathy, but suspicious supraciliary effusion was noted in both eyes. The axial length as measured by ultrasound was 16.0 and 16.2 mm on the right and left eyes, respectively. The sclera had a thickness greater than 2.25 mm on both sides. Cataract surgery was performed on each eye with one week between the two surgeries. The patient was followed-up for two months in the clinic and then did not present to the clinic for two years. He subsequently returned to the clinic with a complaint of decreased visual acuity. There was serous subretinal fluid and moderate non-proliferative diabetic retinopathy in the right eye. Drainage sclerotomy was conducted under general anesthesia in the inferotemporal and inferonasal quadrants. The subretinal fluid in the right eye disappeared one month after the surgery. Twelve months after the drainage sclerotomy, we performed panretinal photocoagulation for diabetic retinopathy. However, serous retinal detachment was noted in his left eye at that time, and the same drainage sclerotomy was performed on the left eye. One and a half years after the first surgery on the right eye and six months after the surgery on the left eye, both retinas remained flat. The final BCVA was 0.01 logMAR in both eyes.
UES is a rare condition with two characteristic findings, uveal effusion and serous retinal detachment. The condition is difficult to manage and often follows a relapsing course. Unlike many other conditions that produce uveal effusion, UES is seldom accompanied by significant inflammation, but is closely related to nanophthalmos and a thick sclera. Elagouz et al. [16] reviewed the pathogenesis of UES and reported that some components that induce abnormalities of the sclera could cause UES, including scleral protein permeability, reduced scleral hydraulic conductivity, vortex vein compression, increased choroidal vessel permeability, chronic choroidal inflammation, and chronic hypotony. Three of our five cases were nanophthalmic, and four cases had a thick sclera. Uyama et al. [7] divided UES into three groups based on pathogenesis, axial length of the eye, and scleral thickness. Type 1 was UES in a nanophthalmic eye with an axial length less than 19 mm, high-grade hyperopia, and a thick sclera. Type 2 UES was not associated with nanophthalmos or hyperopia, but had a thick sclera. Although Brockhurst [10] described good surgical results with decompression of vortex veins, other investigators [5,6] have questioned the usefulness of this technique because vortex veins are difficult to isolate. Uyama et al. [7] reported that sclerectomy with a small sclerostomy under the scleral flap could be effective in both type 1 and type 2 UES because the abnormal sclera and increased resistance to the trans-scleral outflow of intraocular fluid are thought to be the main causes of these disorders. However, this technique was not effective in type 3 UES, which develops in non-nanophthalmic eyes with normal eyeball size and normal scleral thickness.
We believe that isolation of the vortex veins is very complicated, and that decompression is technically difficult to perform without complications, such as vein rupture. Therefore, we elected to perform full-thickness sclerotomy without sclerectomy because it seemed likely to have a sufficient effect for resolving uveal effusion [17]. We suspect that sclerotomy may work by two different mechanisms. First, the full-thickness sclerotomy may work in the immediate postoperative period by facilitating the drainage of uveal exudation. However, we found that there was a small amount of fluid drained intraoperatively. Thus, more importantly, it seems to decompress vortex veins indirectly by relaxing the scleral tension. Compared with sclerectomy with a small sclerostomy under the scleral flap, our full-thickness sclerotomy could simplify the operative procedure and reduce operation time and complications, such as bleeding and hypotony. Furthermore, by placing loose sutures at two corners of the rectangle on the full-thickness scleral flap, we reinforced the tectonic force on the flaps to resist minor trauma. In cases 1, 2, 4, and 5, which were either nanophthalmic or had a thick sclera, the effect of the drainage sclerotomy was sufficient to reduce the subretinal fluid and uveal effusion, and the improvement lasted more than six months. Case 3, which would be classified as type 3 by Uyama et al. [7], had improved visual acuity and reattachment of the retina after drainage sclerotomy, and the retina remained attached at the last clinical follow-up.
To summarize, full-thickness drainage sclerotomy resulted in the resolution of UES, including type 3 UES, and a subsequent improvement in visual acuity. The effect may come from indirect decompression of vortex veins by relaxing scleral tension. In conclusion, our data suggests that full-thickness sclerotomy without vortex vein decompression is an effective surgical option for the management of clinically significant UES.
Figures and Tables
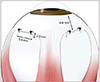 | Fig. 1A square bracket shaped full-thickness scleral incision was made in the quadrant between the rectus muscles. The scleral flap was loosely sutured with 8-0 vicryl at two corners of the rectangle. |
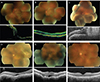 | Fig. 2(A) Preoperative fundus photography of case 1. There are large peripheral choroidal effusions associated with a retinal detachment extending up to the level of the macula. Optical coherence tomography revealed retinal detachment with marked subretinal fluid. (B) Flat retina of case 1 at one month postoperatively. (C) Preoperative fundus photography of case 2. Bullous retinal detachment with macular involvement was noted. (D) The subretinal fluid was reduced at one month after the third surgery in case 2. (E) Preoperative retinal detachment with marked shifting of the subretinal fluid in case 3. (F) Flat retina of case 3 at three months postoperatively. |
References
1. Von Graefe A. Zur diagnose des beginnenden intraocularen krebses. Arch Ophthalmol. 1858; 4:2180–2229.
2. Verhoeff FH, Waite JH. Separation of the choroid, with report of a spontaneous case. Trans Am Ophthalmol Soc. 1925; 23:120–139.
3. Schepens CL, Brockhurst RJ. Uveal effusion. 1. Clinical picture. Arch Ophthalmol. 1963; 70:189–201.
4. Brockhurst RJ. Nanophthalmos with uveal effusion: a new clinical entity. Arch Ophthalmol. 1975; 93:1989–1999.
5. Gass JD. Uveal effusion syndrome: a new hypothesis concerning pathogenesis and technique of surgical treatment. Trans Am Ophthalmol Soc. 1983; 81:246–260.
6. Gass JD, Jallow S. Idiopathic serous detachment of the choroid, ciliary body, and retina (uveal effusion syndrome). Ophthalmology. 1982; 89:1018–1032.
7. Uyama M, Takahashi K, Kozaki J, et al. Uveal effusion syndrome: clinical features, surgical treatment, histologic examination of the sclera, and pathophysiology. Ophthalmology. 2000; 107:441–449.
8. Trelstad RL, Silbermann NN, Brockhurst RJ. Nanophthalmic sclera: ultrastructural, histochemical, and biochemical observations. Arch Ophthalmol. 1982; 100:1935–1938.
9. Forrester JV, Lee WR, Kerr PR, Dua HS. The uveal effusion syndrome and trans-scleral flow. Eye (Lond). 1990; 4(Pt 2):354–365.
10. Brockhurst RJ. Vortex vein decompression for nanophthalmic uveal effusion. Arch Ophthalmol. 1980; 98:1987–1990.
11. Gass JD. Uveal effusion syndrome: a new hypothesis concerning pathogenesis and technique of surgical treatment. Retina. 1983; 3:159–163.
12. Allen KM, Meyers SM, Zegarra H. Nanophthalmic uveal effusion. Retina. 1988; 8:145–147.
13. Casswell AG, Gregor ZJ, Bird AC. The surgical management of uveal effusion syndrome. Eye (Lond). 1987; 1(Pt 1):115–119.
14. Johnson MW, Gass JD. Surgical management of the idiopathic uveal effusion syndrome. Ophthalmology. 1990; 97:778–785.
15. Schneiderman TE, Johnson MW. A new approach to the surgical management of idiopathic uveal effusion syndrome. Am J Ophthalmol. 1997; 123:262–263.
16. Elagouz M, Stanescu-Segall D, Jackson TL. Uveal effusion syndrome. Surv Ophthalmol. 2010; 55:134–145.
17. Faulborn J, Kolli H. Sclerotomy in uveal effusion syndrome. Retina. 1999; 19:504–507.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download