Abstract
Purpose
To assess the clinical outcomes in idiopathic epiretinal membrane (ERM) patients after vitrectomy and ERM removal with or without additional indocyanine green (ICG)-assisted internal limiting membrane (ILM) peeling.
Methods
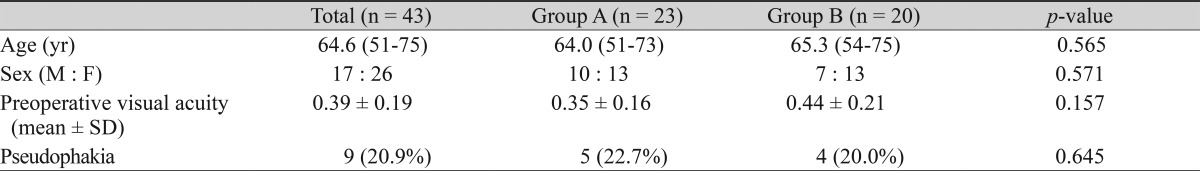
The medical records of 43 patients with an idiopathic ERM that underwent vitrectomy and ERM removal between July 2007 and April 2010 were reviewed. The patients were divided into two groups: triamcinolone-assisted simple ERM peeling only (group A, n = 23) and triamcinolone-assisted ERM peeling followed by ICG staining and peeling of the remaining internal ILM (group B, n = 20).
Results
No difference was found between the two groups in terms of visual acuity, macular thickness, P1 amplitude or implicit time on multifocal-electroretinogram (mfERG) at six and 12 months postoperatively. In group B, ICG staining after ERM peeling demonstrated that the ILM had been removed together with the ERM in 12 eyes (60%), and all 12 eyes showed punctate retinal hemorrhages during ERM peeling. There was no recurrence of an ERM in either group.
Go to : 
An epiretinal membrane (ERM) is a relatively common retinal disorder with a prevalence of 3.5% to 6.9% [1-3]. It can be caused secondarily in various ocular conditions, such as uveitis, trauma, retinal detachment, or retinal vascular diseases. However, causative abnormalities are not found and are considered to be idiopathic in many cases. In these cases, internal limiting membrane (ILM) disruption following posterior vitreous detachment is thought to play an important role in ERM development [4].
Vitrectomy is usually performed to remove the ERM in symptomatic cases. Recurrence after successful surgery has been reported to occur in 10% to 16.3% of cases [5,6]. ILM removal can be performed to reduce the recurrence rate [6], but mechanical trauma to the sensory retina and toxicity by the staining dye is a concern [7].
In the present study, we aimed to compare the clinical results of triamcinolone-assisted simple ERM removal and additional ILM peeling using indocyanine green (ICG) staining in patients with an idiopathic ERM.
This study followed the ethical standards established in the Declaration of Helsinki and was approved by the institutional review board of Inje University Busan Paik Hospital. We retrospectively reviewed the medical records of consecutive patients who underwent vitrectomy for idiopathic ERM between July 2007 and April 2010.
Patients were included in the study if they were followed-up for at least 12 months. The patients with previous history of vitrectomy, other accompanying macular diseases, retinal detachment, retinal vascular disease, endophthalmitis, or diabetic retinopathy were excluded from this study. Initially, 103 eyes of 99 patients who underwent vitrectomy for ERM during the study period were reviewed. Finally, 43 eyes of 43 patients who met the criteria were included for data analysis.
All procedures were performed by a single surgeon (IHY) at the Inje University Busan Paik Hospital, Busan, Korea. A standard 20-gauge pars plan vitrectomy was performed. Phacoemulsification and intraocular lens implantation through a 3.0 mm temporal clear corneal incision was performed before vitrectomy, if indicated.
After core vitrectomy, triamcinolone acetonide (TA) was injected into the vitreous cavity to determine if posterior vitreous detachment had occurred. For cases without posterior vitreous detachment, a pick or a vitreous cutter switched to the vacuum mode was used to induce posterior vitreous detachment. Then, TA was injected onto the ERM to better visualize the boundary of the peeled ERM. Intraocular forceps were used to grasp and remove the ERM.
Since November 2009, we began to use a double staining technique using ICG dye (Diagnogreen; Daiichi Pharmaceutical, Tokyo, Japan) to completely peel the ILM from underneath the ERM. This technique involved injecting 0.5% ICG dye onto the macula right after the previously described procedure to determine if any residual ILM remained. In the cases that it was present, we removed the residual ILM, which was about two disc diameters in size.
Best-corrected Snellen visual acuity and optical coherence tomography (OCT) data were collected at baseline and postoperative 3, 6, and 12 months. Stratus OCT (Carl-Zeiss Meditec, Dublin, CA, USA) or Cirrus HD-OCT (Carl-Zeiss Meditec) was used to acquire central macular thickness.
Preoperative and postoperative multifocal ERG data were also compared. Multifocal ERG was performed using RETIscan (Roland Consult Elektrophysiologische Diagnostik Systeme, Wiesbaden, Germany) following the manufacturer's instructions. Briefly, pupils were maximally dilated, and an ERG jet was used as an electrode. The stimulus consisted of an array of 61 hexagons scaled with eccentricity. The viewing distance was 28 cm, which covers approximately 30° of visual angle. Signals were band pass filtered (5-100 Hz) and amplified (gain, 100,000). The mean simultaneous response component for the first-order kernel was recorded. The averages of responses recorded during eight cycles were calculated for each subject with 61 hexagons and analyzed using the RETIscan software, version 3.15. The amplitude and the latency of the first positive peak (P1) of the ring 1 area were collected for comparison. Perioperative and postoperative complications including punctate retinal hemorrhages during ERM peeling were reviewed.
Patients were divided into two groups. Group A consisted of patients where simple ERM removal using only triamcinolone was performed. Group B consisted of patients where the double staining technique using ICG dye after the TA-assisted ERM peeling and complete ILM peeling was performed. Wilcoxon's signed rank test was used to compare the baseline and the postoperative data at each time point. Mann-Whitney's U-test was used to compare numerical data between the two groups. A p-value of <0.05 was considered statistically significant.
Go to : 
Of the 43 patients included in this study, 17 were male (39.5%) and 26 were female (60.5%). The average age of the patients was 64.6 years (range, 51 to 75 years). Nine eyes (20.9%) were pseudophakic at baseline (Table 1). Vitrectomy alone was performed in five pseudophakic eyes and in 18 eyes with a clear lens in group A and in four and 16 such eyes, respectively, in group B. Combined vitrectomy and cataract surgery was performed in one eye with lens opacity in group A.
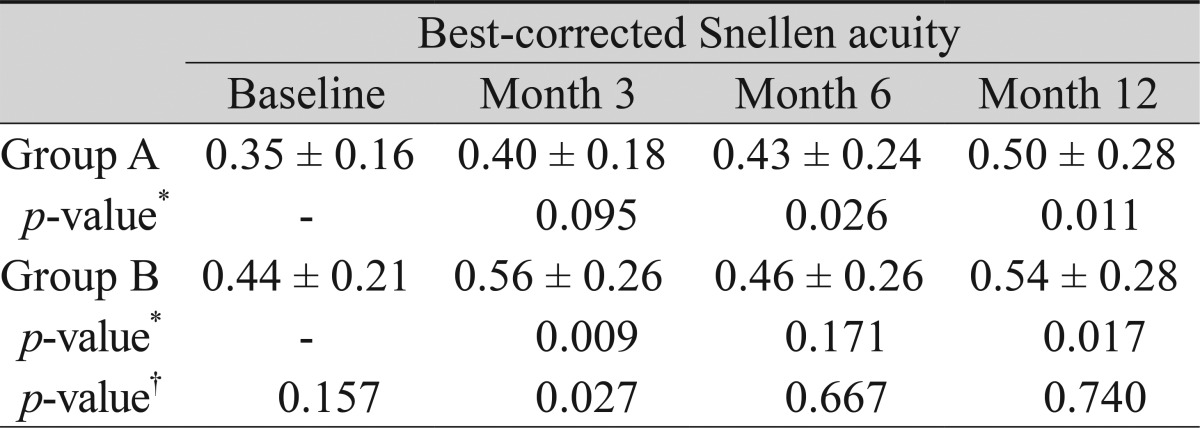
Overall, the mean best-corrected Snellen visual acuity improved from 0.35 logMAR at baseline to 0.52 logarithm of minimum angle resolution (logMAR) at postoperative month 12. This change was significant (p = 0.001). In group A, visual acuity changed from 0.35 logMAR at baseline to 0.40, 0.43, and 0.50 logMAR at postoperative months 3, 6, and 12, respectively, and in group B from 0.44 to 0.56, 0.46, and 0.54 logMAR, respectively. Postoperative visual acuity improvement at postoperative month 12 was statistically significant in both groups (Wilcoxon's signed rank test, p = 0.011 in group A and 0.017 in group B). The difference between the groups at postoperative month 12 was not significant (Mann-Whitney's U-test, p = 0.740) (Table 2).
Decrease in the visual acuity at postoperative month 12 was observed in four patients (two in group A and two in group B). The cause of the decrease in visual acuity was attributed to the development of a cataract. Of the four patients, three received cataract operation and showed an increase in visual acuity of more than two lines compared to the baseline acuity.
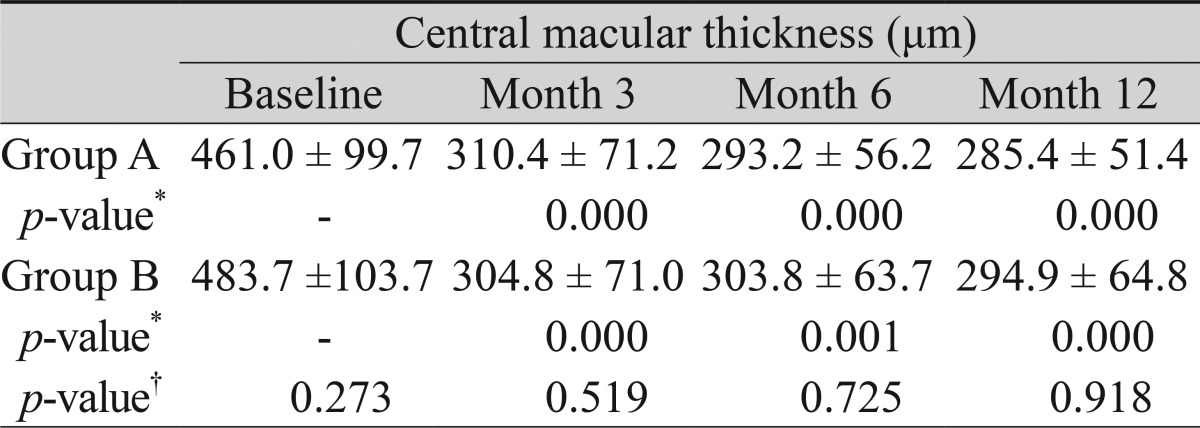
Overall, central macular thickness measured by OCT changed from 471.5 ± 101.0 µm at baseline to 307.8 ± 70.3 µm, 298.0 ± 59.1 µm, and 289.6 ± 57.2 µm at postoperative months 3, 6, and 12, respectively. In group A, it changed from 461.0 ± 99.7 µm at the baseline to 310.4 ± 71.2 µm, 293.2 ± 56.2 µm, and 285.4 ± 51.4 µm at postoperative months 3, 6, and 12, respectively. In group B, it changed from 483.7 ± 103.7 µm at baseline to 304.8 ± 71.0 µm, 303.8 ± 63.7 µm, and 294.9 ± 64.8 µm at postoperative months 3, 6, and 12, respectively (Table 3). The change from baseline to postoperative month 12 was statistically significant in both groups (p = 0.000 in group A and 0.000 in group B). However, the difference between the groups at each time point was not statistically significant.
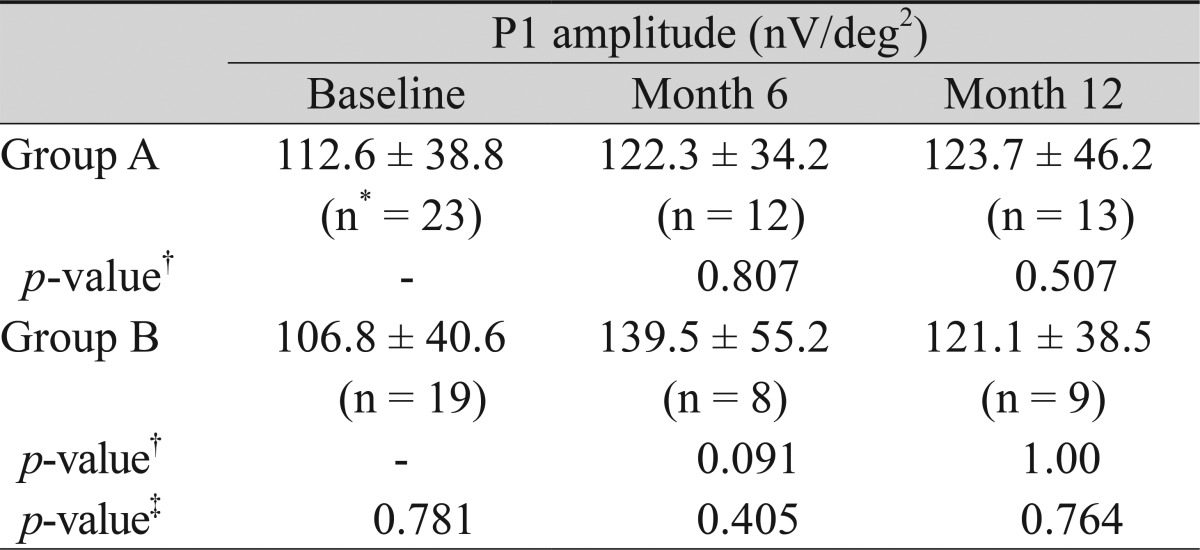
Multofocal-electroretinogram (mfERG) data were available for 23, 12, and 13 patients at baseline and postoperative months 6 and 12, respectively, in group A. In group B, data was available for 19, 8, and 9 patients at baseline and postoperative months 6 and 12, respectively. In group A, the P1 amplitude (nV/deg2) at ring 1 changed from 112.6 ± 38.8 to 122.3 ± 34.2 and 123.7 ± 46.2 at postoperative months 6 and 12, respectively. In group B, it changed from 106.8 ± 40.6 to 139.5 ± 55.2 and 121.1 ± 38.5 at postoperative months 6 and 12, respectively. Comparison of the P1 amplitude within the group at each time point and between the groups did not show any significant differences (Table 4).
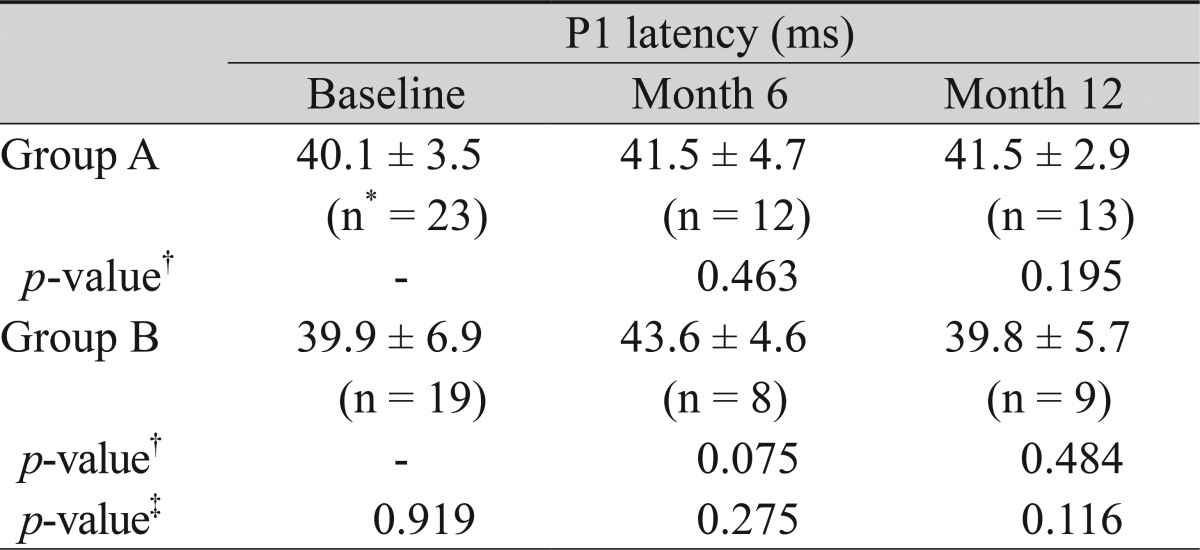
Overall, in group A, the P1 latency (ms) at ring 1 changed from 40.1 ± 3.5 to 41.5 ± 4.7 and 41.5 ± 2.9 at postoperative months 6 and 12, respectively. In group B, it changed from 39.9 ± 6.9 to 43.6 ± 4.6 and 39.8 ± 5.7, respectively. Comparison of the P1 latency within the group at each time point and between the groups did not show any significant differences (Table 5).
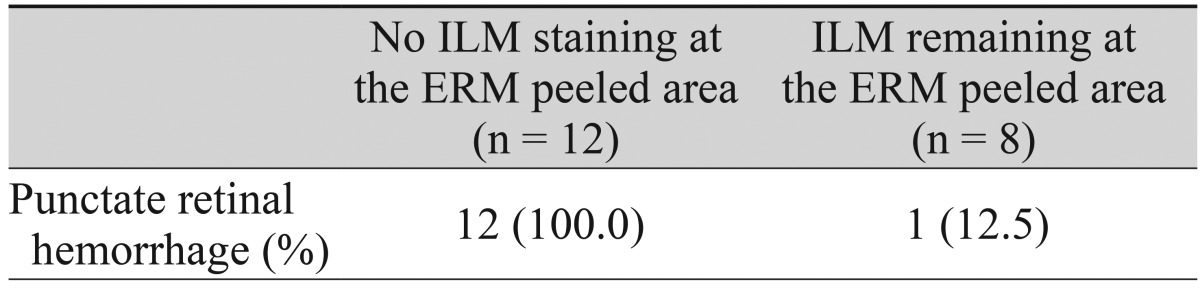
In the 20 eyes from group B in which ICG staining was used to determine if any residual ILM existed after removal of the ERM, 12 eyes (60%) demonstrated no staining of ICG at the site of ERM peeling. This was highlighted by the surrounding ICG stained area.
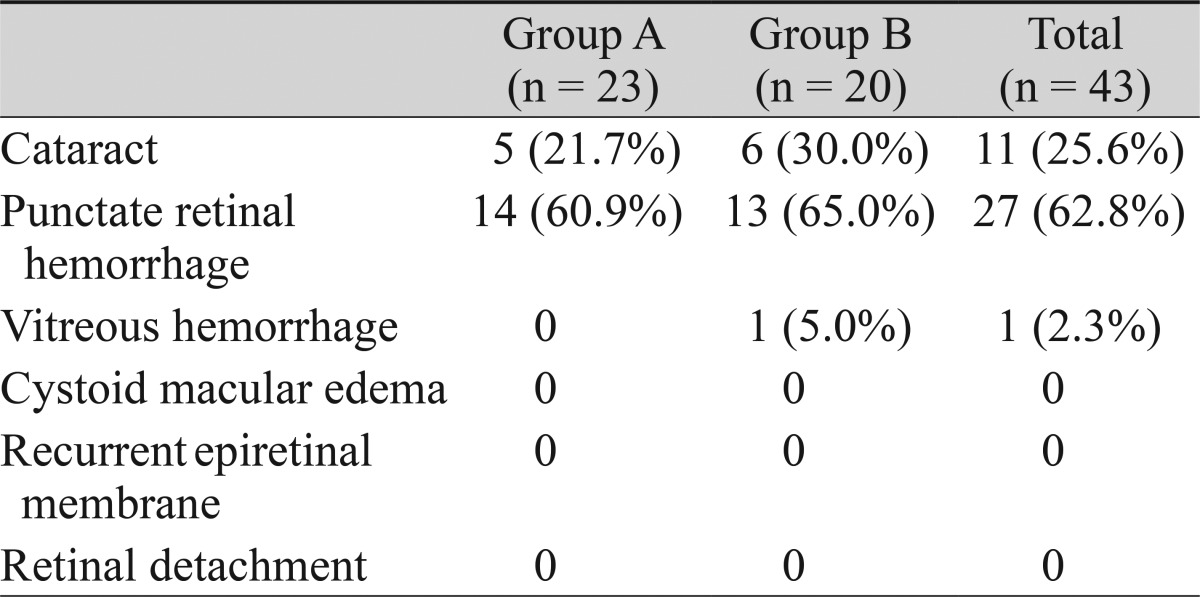
Overall, punctate retinal hemorrhage was observed right after ERM peeling in 27 eyes (62.8%); 14 eyes (60.9%) in group A and 13 eyes (65.0%) in group B. In group B, all 12 eyes in which the ILM had already been removed together with the ERM, as confirmed by ICG double staining, showed punctate retinal hemorrhage (Table 6).
Serious complications including retinal detachment were not observed in either of the groups (Table 7). Vitreous hemorrhage occurred in one patient in group B and was absorbed without sequelae during the first week after surgery. A cataract developed in five and six eyes in groups A and B, respectively. None of the patients in this study exhibited recurrence of the ERM.
Go to : 
Symptomatic ERM is mainly treated by surgical intervention involving vitrectomy and membrane peeling. With advancements in surgical techniques and instrumentation including vital dye that can stain the ILM, many studies have been performed to determine if ILM removal can result in better clinical outcomes [6,8].
Some authors advocate that removal of the ILM can reduce the recurrence rate, because the ILM can act as a scaffold for glial proliferation after successful removal of the ERM [6]. However, long term effects of internal limiting membrane removal are not known, and mechanical injury to the neurosensory retina during ILM peeling has been a concern [9-12]. Toxicity of the vital dye used during the procedure is another concern [7]. Although less toxic and safer dyes have recently been introduced, they are not currently available in many regions [6,13,14].
During removal of the ERM, the internal limiting membrane can be removed together with the ERM in about 40% of cases [6]. The rate of simultaneous removal of both the ERM and the ILM was 60% in the present study.
Here, we aimed to compare the clinical outcomes in two groups: one in which triamcinolone-assisted simple ERM peeling was performed, and the other in which double staining using ICG dye was used to completely peel the ILM from underneath the ERM. Although group A showed significantly better visual acuity at three months, both groups showed comparable results in terms of visual acuity, mfERG change, and complications including recurrence rate at one year. Comparable outcomes between the two groups shown in this study can be explained by the high simultaneous ERM and ILM peeling rate demonstrated in group B, which is likely to be similar in group A. The reason for better visual acuity transiently shown in group A at month 3 is unclear. However, it could be due to better baseline visual acuity that therefore possesses greater visual improvement potential.
Interestingly, punctate retinal hemorrhage was observed during ERM peeling in all patients in group B in which ILM was confirmed to have been removed together with the ERM by the double staining technique. This result shows that punctate retinal hemorrhage can be a good indicator that the ILM beneath the ERM is simultaneously being peeled. Thus, double staining using potentially toxic vital dyes can be reserved only for cases that do not show punctate retinal hemorrhages during ERM peeling.
In conclusion, based on our results, an additional procedure involving ICG staining and ILM peeling does not seem to have additive beneficial effects, at least for the first postoperative year. However, the present study has an inherent weakness because it is a retrospective study, and it has a small number of patients. mfERG data were not available for all the patients during the follow-up period. Only 13 patients in group A and nine patients in group B had available mfERG data at 12 months. Therefore, the results regarding the mfERG data comparison might have not reflected the true outcomes. Also, a follow-up period of 12 months is relatively short to assess the recurrence rate of ERM after vitrectomy and ERM removal. A prospective study on a larger scale and with longer follow-up is warranted.
Go to : 
References
1. Kawasaki R, Wang JJ, Sato H, et al. Prevalence and associations of epiretinal membranes in an adult Japanese population: the Funagata Study. Eye (Lond). 2009; 23:1045–1051. PMID: 19440207.

2. Klein R, Klein BE, Wang Q, Moss SE. The epidemiology of epiretinal membranes. Trans Am Ophthalmol Soc. 1994; 92:403–425. PMID: 7886875.
3. Mitchell P, Smith W, Chey T, et al. Prevalence and associations of epiretinal membranes. The Blue Mountains Eye Study, Australia. Ophthalmology. 1997; 104:1033–1040. PMID: 9186446.
4. Smiddy WE, Maguire AM, Green WR, et al. Idiopathic epiretinal membranes: ultrastructural characteristics and clinicopathologic correlation. Ophthalmology. 1989; 96:811–820. PMID: 2740079.
5. Grewing R, Mester U. Results of surgery for epiretinal membranes and their recurrences. Br J Ophthalmol. 1996; 80:323–326. PMID: 8703883.

6. Shimada H, Nakashizuka H, Hattori T, et al. Double staining with brilliant blue G and double peeling for epiretinal membranes. Ophthalmology. 2009; 116:1370–1376. PMID: 19427701.

7. Lee JE, Yoon TJ, Oum BS, et al. Toxicity of indocyanine green injected into the subretinal space: subretinal toxicity of indocyanine green. Retina. 2003; 23:675–681. PMID: 14574254.
8. Park DW, Dugel PU, Garda J, et al. Macular pucker removal with and without internal limiting membrane peeling: pilot study. Ophthalmology. 2003; 110:62–64. PMID: 12511347.

9. Sivalingam A, Eagle RC Jr, Duker JS, et al. Visual prognosis correlated with the presence of internal-limiting membrane in histopathologic specimens obtained from epiretinal membrane surgery. Ophthalmology. 1990; 97:1549–1552. PMID: 2255528.
10. Smiddy WE, Feuer W, Cordahi G. Internal limiting membrane peeling in macular hole surgery. Ophthalmology. 2001; 108:1471–1476. PMID: 11470703.

11. Shea M. The surgical management of macular pucker in rhegmatogenous retinal detachment. Ophthalmology. 1980; 87:70–74. PMID: 7375088.

12. Terasaki H, Miyake Y, Nomura R, et al. Focal macular ERGs in eyes after removal of macular ILM during macular hole surgery. Invest Ophthalmol Vis Sci. 2001; 42:229–234. PMID: 11133873.
13. Mochizuki Y, Enaida H, Hisatomi T, et al. The internal limiting membrane peeling with brilliant blue G staining for retinal detachment due to macular hole in high myopia. Br J Ophthalmol. 2008; 92:1009. PMID: 18577657.

14. Enaida H, Ishibashi T. Brilliant blue in vitreoretinal surgery. Dev Ophthalmol. 2008; 42:115–125. PMID: 18535385.

Go to : 




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download