Abstract
Purpose
To investigate the morphologic changes in the outer retina of patients with cone dystrophy, using spectral-domain optical coherence tomography (SD-OCT).
Methods
The medical records of 15 cone dystrophy patients examined from January 2007 to January 2012 were reviewed retrospectively. All patients underwent ophthalmic evaluation including best-corrected visual acuity (BCVA), color vision testing, fundus examination, full-field standard electroretinography (ERG), multifocal (mf) ERG, and SD-OCT. Qualitative and quantitative SD-OCT data and ERG responses were analyzed and compared among the patient categories and the normal control group.
Results
There were 4 major categories of SD-OCT findings, based on the status of the ellipsoid portion of the photoreceptor inner segment (ISe), outer segment (OS) contact cylinder, and retinal pigment epithelium (RPE) layer. Category 0 showed no structural abnormalities. Category 1 showed foveal ISe loss and obscurity of the border between the ISe band and the external limiting membrane (ELM). Category 2 showed foveal thinning and focal foveal ISe disruption with an intact ELM. Category 3 showed foveal thickening and perifoveal disruption of the ISe layer. Category 1 to 3 showed OS contact cylinder layer absence and RPE thickening. The patients in category 0 tended to be younger (mean, 10.0 years) than those in categories 1 to 3 (mean, 17.6 years), although this difference was not statistically significant. Category 1 to 3 patients exhibited statistically significant thinning of the central retina and outer nuclear layer and thickening of the RPE layer relative to the category 0 and normal control group. There was a significant correlation between the central foveal thickness and BCVA in the patients with cone dystrophy. ERG and mfERG responses did not differ significantly among the different cone dystrophy categories.
Conclusions
The morphologic features of cone dystrophy as revealed by SD-OCT, could be categorized as either normal or 1 of 3 different types of outer retinal changes. The presence of normal retinal structures in young cone dystrophy patients with functional impairment (category 0) indicates that electrophysiologic studies are superior to current imaging modalities for the early diagnosis of cone dystrophy. The characteristic SD-OCT findings in cone dystrophy patients may aid in differential diagnosis and be useful for future research on the pathology of cone dystrophy.
Cone dystrophy is an inherited retinal disorder characterized by the loss of cone cells- the photoreceptors responsible for central and color vision. The most common symptoms are progressive vision loss, poor color vision, and sensitivity to bright light. Full-field electroretinography (ERG), which demonstrates reduced single-flash and flicker responses in the photopic condition and a normal response in the scotopic condition, is the gold-standard test.
The widespread adoption of high-resolution spectral-domain optical coherence tomography (SD-OCT) enabled investigators to demonstrate structural abnormalities in the retinal layers and their correlations with functional status in various retinal diseases, such as acute zonal occult outer retinopathy-complex diseases [1], epiretinal membrane [2], macular hole [3,4], and retinitis pigmentosa [5]. Several investigators have studied the retinal structural changes in cone dystrophy patients using SD-OCT. Birch et al. [6] evaluated the effects of selective rod or cone cell loss on SD-OCT findings in patients with cone dystrophy or retinitis pigmentosa and reported that the normalized product of the thickness of the outer nuclear layer (ONL) and the summed thickness of the photoreceptor outer segment (OS) and retinal pigment epithelium (RPE) was proportional to the visual field sensitivity. Hood et al. [7] studied patients with cone dystrophy or achromatopsia and showed that the intensity of the inner segment ellipsoid (ISe) band, previously called the inner segment (IS)-OS junction band, of the photoreceptor seen by SD-OCT was lower in patients with diminished cone function than in healthy controls. Each of those studies included only 6 patients with cone dystrophy, which is a relatively small number, and did not show the morphologic SD-OCT features of the outer retina in detail.
In the present study, we attempted to analyze the structural changes in the outer retinas of 15 patients with cone dystrophy using SD-OCT and related the structural changes to the functional and electrophysiologic findings.
The medical records of 15 patients diagnosed with cone dystrophy from January 2007 to January 2012 were retrospectively reviewed. The diagnostic criteria for cone dystrophy are as follows; a substantial decrease in cone cell function with relative preservation of rod cell function, which can be demonstrated by a full-field ERG, and a history of progressive visual loss, photophobia, and poor color vision (Fig. 1).
All of the patients underwent full ophthalmic evaluation including best corrected visual acuity test, manifest or cycloplegic refraction examination, color vision testing, slit lamp examination, fundus examination, full-field standard ERG, multifocal ERG (VERIS II; Electro-Diagnostic Imaging Inc., San Francisco, CA, USA), and SD-OCT (Spectralis OCT; Heidelberg Engineering, Heidelberg, Germany). Some of the patients also underwent visual field testing by Humphrey or Goldmann perimetry, fluorescein angiography, and fundus autofluorescence imaging.
The retinal structural changes on the SD-OCT images were analyzed and the findings were compared between the patients and the normal control group. The normal control group was comprised of individuals who were diagnosed as normal based on the results of ophthalmic examination including SD-OCT. The 4 hyper-reflective bands in the outer retina visible on SD-OCT images were analyzed qualitatively based on the model proposed by Spaide and Curcio [8]. The first (innermost) band corresponds to the external limiting membrane (ELM), the second (beneath the first band) to the ellipsoid portion of the inner segments (ISe), the third band (beneath the ISe band) to a structure known as the OS contact cylinder, which is an ensheathment of the cone outer segments by an apical process of the RPE, and the last, outermost band to the RPE (Fig. 2A). A retina specialist (SJW) and an ophthalmologist (SCC) thoroughly evaluated the SD-OCT images of all 30 eyes from the 15 patients.
The quantitative data included the thickness of the retinal layers as measured by SD-OCT and the ERG amplitudes. The retinal thickness profiles included the central foveal thickness (CFT) and the thickness of the ONL, photoreceptor (PR) and RPE (PR + RPE; defined as the distance between the outer margin of the RPE and the outer margin of the ELM), and RPE. The ERG data included the full-field standard ERG amplitude (scotopic maximal response, photopic response, and 30-hertz flicker response) and the multifocal ERG innermost ring amplitude.
The eyes were classified into 4 different categories based on the morphologic retinal abnormalities on the SD-OCT images, and the qualitative and quantitative data were compared between the patients and the normal control group.
The Mann-Whitney U-test was used for inter-group comparison of the quantitative data. Student's t-test was used to compare the quantitative data between the total patient group and the normal control group. Statistical analysis was performed using commercial software (SPSS ver. 18.0; SPSS Inc., Chicago, IL, USA). A p-value less than 0.05 was regarded to be statistically significant.
Seven of the 15 patients were men and 8 were women. The mean (±SD) age was 15.6 ± 12.7 years (range, 5 to 46 years). The best-corrected visual acuities (BCVA) of the patients' eyes ranged from 20 / 500 to 20 / 30. All patients showed ERG findings consistent with cone dystrophy. No patient had any afferent pupillary defect or visual pathway abnormality.
Of the 5 patients who had undergone Humphrey perimetry, 1 had deep central scotomas within the central 5 degrees of fixation of both eyes (case 4). Another patient had a unilateral central scotoma in the right eye and a generalized reduction of sensitivity in the left eye (case 9) (Fig. 1G and 1I). Three patients had bilateral reduction of sensitivity in the central visual field (cases 3, 8, and 14).
SD-OCT demonstrated morphologic changes in the outer retinal layers. Of the 30 eyes of the 15 patients, 22 eyes (of 11 patients) showed changes in the outer retinal layers in both eyes. Another 8 eyes (in 4 patients) showed no definite structural changes on OCT. There were 4 major categories of SD-OCT findings, based on the status of the ISe band, OS contact cylinder layer, and RPE layer. Both eyes for each individual showed symmetric categorization. The first category (category 0: cases 5, 6, 10, and 13) (Fig. 2B) showed normal SD-OCT findings. The second category (category 1: cases 1, 2, 8, and 9) (Fig. 2C) showed irregular foveal loss of the ISe band and obscurity of the border between the ISe band and the ELM due to the accumulation of reflective material between the 2 layers. The third category (category 2: cases 3, 7, 11, 12, 14, and 15) (Fig. 2D) showed retinal thinning around the fovea and segmental (or irregular) foveal loss of the ISe band. The last category (category 3: case 4) (Fig. 2E) showed ISe band thickening around the fovea and irregular perifoveal loss of the ISe band. In category 1 to 3, the OS contact cylinder layer was not discernible and the RPE layer was thickened (Fig. 2).
The demographic, quantitative, and qualitative SD-OCT data for the patients and the normal control group are shown in Tables 1 and 2, and Fig. 3. The patients in category 0 tended to be younger (mean, 10.0 years) than those in categories 1 to 3 (mean, 17.6 years), although this difference was not statistically significant (Mann Whitney U-test, p = 0.317). The CFT, ONL, PR + RPE and PRE thicknesses did not differ significantly between the normal control group and category 0 patients. CFT, ONL, and PR+RPE thicknesses were significantly lower in category 1 to 3 patients than in the normal control group or the cone dystrophy category 0 patients. It is noteworthy that the RPE (the outermost high signal band) thickness was greater in category 1 to 3 patients than in the normal control group or the category 0 patients. The CFT was significantly lower in category 2 patients than in category 1 or 3 patients (p < 0.001 and p = 0.022, respectively).
To investigate the structure-function relationship in cone dystrophy patients, we compared the BCVA among the patient categories (Fig. 4). The mean BCVAs of category 1 and 2 patients were significantly worse than that of category 0 patients (p = 0.031 and p = 0.006, respectively). CFT and BCVA correlated significantly in cone dystrophy patients (Spearman's correlation coefficient = 0.648, p < 0.001).
In the standard full-field ERG data, the scotopic maximal response did not differ significantly between cone dystrophy patients and the normal control group (Fig. 5A). The photopic and 30-Hz flicker responses were lower in the cone dystrophy category 0 to 3 patients than in the normal control group (p < 0.001) (Fig. 5B and 5C). The scotopic maximal, photopic, and 30-Hz flicker responses did not differ among patient categories (category 0 to 3). CFT and photopic response correlated significantly in cone dystrophy patients (Spearman's correlation coefficient = 0.371, p = 0.043). Although the correlation between CFT and 30-Hz flicker response was not statistically significant (p = 0.094), ONL thickness and 30-Hz flicker response correlated significantly in cone dystrophy patients (Spearman's correlation coefficient = 0.375, p = 0.041).
The multifocal ERG innermost ring amplitude was significantly lower in category 0 patients than in the normal control group (p = 0.005) but did not differ significantly among the patient categories (Fig. 5D).
This study described in depth and categorized the outer retinal morphologic abnormalities in cone dystrophy patients using SD-OCT. To our knowledge, this is the first such report. In our study, structural abnormalities of the outer retinal layers on SD-OCT images were found in 73.3% (11 / 15) of the cone dystrophy patients, and the morphologic features on SD-OCT could be categorized into 4 distinct patterns. With the exception of category 0, which demonstrated no structural changes on SD-OCT, absence of the OS contact cylinder layer and thickening of the RPE layer were found in all of the patient categories, indicating that the reflectivity of the 2 layers was merged due to structural damage to the corresponding layers. This finding also suggests that the retinal pathology of cone dystrophy occurs mainly between the photoreceptor outer segment and RPE layer.
Eyes with cone dystrophy showed decreased CFT due to thinning of the outer retinal layers. In addition, thinning of the central retinal layers correlated with visual acuity and electrophysiologic function in eyes with cone dystrophy. Lim et al. [9] demonstrated that eyes with retinal dystrophy (cone-rod dystrophy, retinitis pigmentosa, and cone-rod degeneration) had a small (11%) decrease in the thickness of the macular inner retinal layer (inner plexiform layer, ganglion cell layer, and nerve fiber layer) and a severe (45%) decrease in the thickness of the macular outer retinal layer, which is consistent with our results. Choi et al. [10] used an adaptive optics fundus camera to demonstrate extensive space between cone cells in patients with retinal dystrophy (rod-cone dystrophy, cone-rod dystrophy, and juvenile macular dystrophy), which is also consistent with our finding that the photoreceptor layer is the major site of structural impairment on SD-OCT.
Although category 0 patients showed no retinal structural abnormalities on SD-OCT, they exhibited electrophysiologic dysfunction comparable to that of patients in the other categories of cone dystrophy. The young age of this group implies that their structural abnormality can not be visualized on SD-OCT in this stage despite the significant functional expression. This finding indicates that ERG is more sensitive and can be used to diagnose cone dystrophy earlier than current SD-OCT techniques. Further research is necessary, as future imaging modalities such as the adaptive optics technique may be able to detect structural abnormalities in category 0 patients.
Category 1 patients showed irregular foveal ISe band disruption and obscurity of the border between the ISe layer and the ELM. Kim et al. [11] reported a similar structural abnormality in a 13-year-old girl with cone-rod dystrophy 6 with mutations in the retinal guanylate cyclase gene (GUCY2D). They demonstrated similar irregular photoreceptor loss at the IS-OS junction (ISe) and in the OS (OS contact cylinder) layer, and showed that the photoreceptor abnormalities on SD-OCT corresponded to the perifoveal ring seen by autofluorescence imaging of the patient. Querques et al. [12] also showed similar disruption and focal loss of the IS-OS junction in the case of a 47-year-old woman with fundus albipunctatus associated with cone dystrophy.
Category 2 patients revealed distinct foveal thinning and segmental loss or irregular disruption of the ISe layer. The central foveal thickness was lower in this category than in any of the other patient categories. Segmental ISe layer defects were previously reported by Hood et al. [7] in a study of cone dystrophy and achromatopsia. Emfietzoglou et al. [13] also reported a similar total absence of the IS-OS junction layer (ISe layer) and foveal thinning in the case of a 25-year-old man with cone-rod dystrophy.
Category 3 patients demonstrated foveal ISe layer thickening. This type of morphologic abnormality was found only in case 4. The accumulation of material consequent to cone cell dysfunction could be the reason for the ISe thickening. This type of structural change was reported by Duncan et al. [14] in a study of cone-rod dystrophy patients with peripherin/RDS mutations.
We showed that the outer retinal SD-OCT findings of cone dystrophy patients are heterogeneous; this diverse pathology may stem from genetic diversity. Several researchers have suggested that cone dystrophy arises from diverse genetic sources with considerable variability in clinical consequences [15]. It is noteworthy that patients in different categories of morphologic abnormality showed similar electrophysiologic responses. This important result indicates that the morphologic characteristics demonstrated by SD-OCT could possibly distinguish patients with different genotypes, while the electrophysiologic study could not. Further genetic study must be performed to test this hypothesis.
In addition to cone dystrophy, some diseases such as acute zonal occult outer retinopathy (AZOOR) complex and occult macular dystrophy (OMD) also show abnormalities on OCT and electrophysiologic test. AZOOR complex is a disease that includes acute idiopathic blind-spot enlargement syndrome, multiple evanescent white dot syndrome, punctate inner choroidopathy, multifocal choroiditis and panuveitis, and acute macular neuroretinopathy. AZOOR complex is similar to cone dystrophy in that the IS/OS boundary defect is found in the OCT. (In a study by Spaide et al. [1] for the 18 eyes with AZOOR complex, patients with blind spot enlargement had outer retinal damage surrounding the optic nerve head while those without blind spot enlargement did not.) Unlike in cone dystrophy, the main symptoms in AZOOR complex are acute loss of one or more zones of visual field and photopsia. In cone dystrophy, only cone response diminishes in the standard full-field ERG while diverse ERG findings are observed in AZOOR complex cases (In a study by Gass et al. [16] for the 90 eyes with AZOOR, both scotopic and photopic function were abnormal in 55 eyes, only the photopic function was abnormal in 16 eyes, and only the scotopic function was abnormal in 16 eyes.) OMD is similar to cone dystrophy in that disruption of the IS/OS junction is observed in the OCT [17]. However, OMD is different from cone dystrophy in that while the standard full-field ERG is normal, the foveal amplitude of the multifocal ERG is decreased.
The limitation of our study is the lack of genotypic data in the enrolled patients. As stated above, the distinct patterns of structural abnormality may represent different patient genotypes. We recently began a genetic study on cone dystrophy patients, and we hope that the genotype-phenotype relationship will be revealed in the near future.
In conclusion, the structural findings on SD-OCT images in cone dystrophy could be classified into 4 major categories based on the morphologic changes in the retinal outer layer. The presence of normal retinal structures in young patients with functional impairment indicates that electrophysiologic studies are superior to current imaging modalities for the early diagnosis of cone dystrophy. The characteristic SD-OCT findings in cone dystrophy patients may aid in differential diagnosis and be useful for future research on the pathology of cone dystrophy.
Figures and Tables
Fig. 1
A 21-year-old man (case 9) with cone dystrophy. (A,B) Fundus photographs showing focal degeneration around the fovea in both eyes. (C,D) Fundus fluorescein angiography showing normal angiographic patterns in the (C) early and (D) late phases. (E,F) Optical coherence tomography showing irregular loss of the ellipsoid portion of the photoreceptor inner segment (ISe) in both eyes. (G,I) Visual field tests by Humphrey field analyzer central 24-2 threshold testing showing a deep central scotoma in the (G) right eye and generalized reduction of sensitivity in the (I) left eye. (H,J) Full-field standard electroretinography showing decreased amplitudes of the photopic (graph of the fourth row) and 30-Hz flicker response (graph of the fifth row) in both eyes. (K,L) Trace arrays of multifocal electroretinography showing decreased central retinal responses in both eyes.
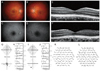
Fig. 2
Spectral-domain optical coherence tomography (SD-OCT) horizontal scan images of a normal control eye (A) and representative cases of each category of cone dystrophy patients (B-E). (A) SD-OCT of normal eyes showed 4 distinct high-signal bands in the outer retinal layer OS. (B) Category 0, OS, case 6, with normal SD-OCT findings. (C) Category 1, OD, case 8, with irregular foveal loss of the ellipsoid portion of the inner segment (ISe) band and obscurity of the border between the ISe and external limiting membrane. (D) Category 2, OS, case 3, with central retinal thinning and segmental foveal loss of the ISe band. (E) Category 3, OD, case 4, with central foveal thickening of the ISe band and irregular perifoveal loss of the ISe band. Loss of the outer segment contact cylinder layer and the thickening of the retinal pigment epithelium layer were found in category 1 to 3 patients.
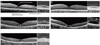
Fig. 3
Optical coherence tomography thickness in the normal control and cone dystrophy grouped categories. (A,B,C) Central foveal thickness (CFT), outer nuclear layer (ONL), and photoreceptor (PR) + retinal pigment epithelium (RPE) thicknesses were significantly lower in category 1 to 3 eyes than in normal control or category 0 eyes. (D) RPE thickness was greater in category 1 to 3 eyes than in normal control or category 0 eyes. The open circles reveal the means of the thicknesses, and the vertical error bars represent the standard deviation. The asterisk indicates a statistically significant difference in thickness (p < 0.05).

Fig. 4
Best-corrected visual acuity (BCVA) in the cone dystrophy category. The open circles reveal the means of the BCVA, and the vertical error bars represent the standard deviation. The asterisk indicates a statistically significant difference in BCVA (p < 0.05). logMAR = logarithm of the minimum angle of resolution.

Fig. 5
Full-field and multifocal electroretinography (mfERG) amplitudes in the normal control and cone dystrophy grouped categories. (A) Scotopic maximal response. (B) Photopic response. (C) 30-Hz flicker response. (D) mfERG ring 1 amplitude. The open circles reveal the means of the amplitudes and the vertical error bars represent the standard deviation. The asterisk indicates a statistically significant difference in amplitude (p < 0.05).

Table 2
Comparison of OCT and ERG data between cone dystrophy categories and normal controls

Values are presented as number (%) or mean ± SD.
SD-OCT = spectral-domain optical coherence tomography; ERG = electroretinography; logMAR = logarithm of the minimum angle of resolution; ISe = ellipsoid portion of photoreceptor inner segment; ELM = external limiting membrane; CFT = central foveal thickness; ONL = outer nuclear layer; PR = photoreceptor; RPE = retinal pigment epithelium.
*Indicates a statistically significant difference compared with the normal control group (p < 0.05).
References
1. Spaide RF, Koizumi H, Freund KB. Photoreceptor outer segment abnormalities as a cause of blind spot enlargement in acute zonal occult outer retinopathy-complex diseases. Am J Ophthalmol. 2008. 146:111–120.
2. Inoue M, Morita S, Watanabe Y, et al. Inner segment/outer segment junction assessed by spectral-domain optical coherence tomography in patients with idiopathic epiretinal membrane. Am J Ophthalmol. 2010. 150:834–839.
3. Oh J, Smiddy WE, Flynn HW Jr, et al. Photoreceptor inner/outer segment defect imaging by spectral domain OCT and visual prognosis after macular hole surgery. Invest Ophthalmol Vis Sci. 2010. 51:1651–1658.
4. Chang LK, Koizumi H, Spaide RF. Disruption of the photoreceptor inner segment-outer segment junction in eyes with macular holes. Retina. 2008. 28:969–975.
5. Hood DC, Lazow MA, Locke KG, et al. The transition zone between healthy and diseased retina in patients with retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2011. 52:101–108.
6. Birch DG, Wen Y, Locke K, Hood DC. Rod sensitivity, cone sensitivity, and photoreceptor layer thickness in retinal degenerative diseases. Invest Ophthalmol Vis Sci. 2011. 52:7141–7147.
7. Hood DC, Zhang X, Ramachandran R, et al. The inner segment/outer segment border seen on optical coherence tomography is less intense in patients with diminished cone function. Invest Ophthalmol Vis Sci. 2011. 52:9703–9709.
8. Spaide RF, Curcio CA. Anatomical correlates to the bands seen in the outer retina by optical coherence tomography: literature review and model. Retina. 2011. 31:1609–1619.
9. Lim JI, Tan O, Fawzi AA, et al. A pilot study of Fourier-domain optical coherence tomography of retinal dystrophy patients. Am J Ophthalmol. 2008. 146:417–426.
10. Choi SS, Doble N, Hardy JL, et al. In vivo imaging of the photoreceptor mosaic in retinal dystrophies and correlations with visual function. Invest Ophthalmol Vis Sci. 2006. 47:2080–2092.
11. Kim BJ, Ibrahim MA, Goldberg MF. Use of spectral domain OCT to visualize photoreceptor abnormalities in cone/rod dystrophy-6. Retin Cases Brief Rep. 2011. 5:56–61.
12. Querques G, Carrillo P, Querques L, et al. High-definition optical coherence tomographic visualization of photoreceptor layer and retinal flecks in fundus albipunctatus associated with cone dystrophy. Arch Ophthalmol. 2009. 127:703–706.
13. Emfietzoglou I, Grigoropoulos V, Nikolaidis P, et al. Optical coherence tomography findings in a case of cone-rod dystrophy. Ophthalmic Surg Lasers Imaging. 2010. 41 Online:e1–e3.
14. Duncan JL, Talcott KE, Ratnam K, et al. Cone structure in retinal degeneration associated with mutations in the peripherin/RDS gene. Invest Ophthalmol Vis Sci. 2011. 52:1557–1566.
15. Thiadens AA, Phan TM, Zekveld-Vroon RC, et al. Clinical course, genetic etiology, and visual outcome in cone and cone-rod dystrophy. Ophthalmology. 2012. 119:819–826.
16. Gass JD, Agarwal A, Scott IU. Acute zonal occult outer retinopathy: a long-term follow-up study. Am J Ophthalmol. 2002. 134:329–339.
17. Park SJ, Woo SJ, Park KH, et al. Morphologic photoreceptor abnormality in occult macular dystrophy on spectral-domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2010. 51:3673–3679.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download