Abstract
Purpose
To report the clinical features, clinical course, and treatment outcomes after laser photocoagulation in infants with aggressive posterior retinopathy of prematurity (APROP) and capillary-free zones in vascularized retina.
Methods
Six patients (12 eyes) with APROP and capillary-free zones in vascularized retina were retrospectively reviewed. Twelve eyes of six infants were included and were treated with laser photocoagulation for avascular retina and for capillary-free zones in vascularized retina, except for the posterior pole, and fundus findings were photographically-documented in sequence. In addition, anatomic and visual outcomes were evaluated with complications of APROP.
Results
Among all of the consecutive infants with APROP, capillary-free zones in vascularized retina were demonstrated in 24% of the infants. All of the infants were >27 weeks of gestation age and had birth weights >1,000 g. After laser treatment, 7 eyes (58.3%) had favorable outcomes, and late capillary filling in capillary-free zones of vascularized retina were noted, however 4 eyes (33.3%) progressed to retinal detachment and 1 eye (8.3%) was complicated by a retinal fold-distorting posterior pole. The visual outcomes were associated with anatomic outcomes.
Conclusions
The anatomic outcomes in infants with APROP who had capillary-free zones were comparable to previously reported infants with APROP. The late capillary filling of capillary-free zones in vascularized retina was noted, and angiogenesis was considered to be involved. This process toward normal capillary formation or neovascularization in APROP, might determine its outcome.
In infants with retinopathy of prematurity (ROP), the retina is characterized by vascular and avascular areas. The border of the vascular and avascular retina demonstrates typical findings, for which the stage of ROP is determined [1]. In the vascular retina of infants with ROP, normal formation of capillaries is noted, while large vessels may exhibit dilation and tortuosity, which signifies the presence of plus disease [1,2]. Observation until threshold or until high-risk pre-threshold is recommended and treatment for these types of ROP includes laser photocoagulation performed on an avascular retina.
Several reports have noted an unusual presentation, including vascular findings and an atypical clinical course compared to classic ROP [1]. Previously, these cases were referred to as type II ROP or rush-type ROP [3,4]. In the 2005 revised report of the International Committee for the Classification of Retinopathy of Prematurity [1], type II ROP or rush-type ROP are termed aggressive posterior retinopathy of prematurity (APROP). This type of eye disease has the following characteristics: 1) more posterior location; 2) rapid progression, rather than through the classic stages 1 to 5; and 3) poor prognosis despite early treatment [1,5-8].
An earlier report highlighted the capillary-free zones in vascularized retina of infants with APROP [7]. This finding is unique in infants with APROP and might be considered a characteristic finding [7]. A previous report advocated laser treatment of these capillary-free zones, including the vascularized retina [7,9]. However, the clinical course and outcomes in infants with APROP who have this distinctive feature have not been evaluated. Thus the clinical significance of capillary-free zones and their implication on the pathogenesis of APROP have yet to be discussed.
Therefore, we sought to determine the clinical features and course of infants who had capillary-free zones in vascularized retina by using serial photographic documentation and also evaluated their treatment outcomes. Using these novel findings, we discuss the underlying mechanism and suggest an appropriate treatment approach.
A retrospective review of infants with APROP was performed, and the infants who demonstrated capillary-free zones in vascularized retina were included in this study. The infants' demographics, including gestational period, gender, and birth weight were investigated, along with systemic diseases and the performance of oxygen therapy. The institutional review board of Seoul National University Hospital approved this study.
All infants underwent initial ROP screening and follow-up examinations guided by previously reported recommendations [10]. After APROP was noted on funduscopic examination, fundus photographs were obtained serially using a RetCam (Massie Research Laboratories Inc., Pleasanton, CA, USA) at the initial and follow-up examinations.
Laser treatment was performed in a near confluent pattern, and the timing of the treatment was within 24 hours after APROP was noted, even though threshold disease was not recognized. In addition to avascular retina, capillary-free zones were treated with laser photocoagulation, except for the posterior pole. In infants with an avascular area uncovered with confluent laser burns by the first laser treatment, additional laser treatments were performed. The post-conceptual age at the time of first and second laser treatments was also reviewed. After the treatment, follow-up examinations were performed weekly until the infant was discharged from the neonatal intensive care unit. The capillary-free zones in vascularized retina were meticulously inspected for any changes on follow-up examinations.
At the final visit, fundus photographs were obtained, and complete ophthalmic examinations were performed to evaluate anatomic and visual outcomes with complications. Anatomically-favorable outcomes were defined as the normal formation of a posterior pole without retinal distortion, whereas an unfavorable outcome included retinal detachment or a retinal fold-distorting posterior pole. The visual outcome was measured by the best-corrected visual acuity (BCVA) in patients who could read a visual acuity chart or by the presence of fix-and-follow behavior in patients who could not read the chart due to young age or mental retardation.
Among the 25 infants with APROP who were examined and diagnosed between July 2000 and May 2010 at Seoul National University Children's Hospital, 6 infants (24%) had capillary-free zones in vascularized retina. Among the six infants, Table 1 demonstrated the funduscopic findings and incidence. Other funduscopic findings included retinal vessels limited to zone I (100%), prominent plus sign (100%), retinal or pre-retinal hemorrhage (50%), and focal ridge or extra-retinal fibrovascular proliferation (33%).
For the six infants with APROP who demonstrated capillary-free zones in vascularized retina, the demographic characteristics including age, gender, gestational age, birth weight, and any systemic diseases are summarized in Table 2. The mean gestational age at birth was 30.5 weeks with a range of 27.5 to 35.4 weeks, and the mean birth weight was 1,432 g (range, 1,120 to 1,960 g). All of the infants in this study were >27 weeks of gestation, and their birth weights were >1,000 g. Except for 1 infant, all others in this study received oxygen therapy, mostly for respiratory distress syndrome (67%); no other co-existing systemic diseases were present.
The capillary-free zones in vascularized retina are shown in Fig. 1. The capillary-free zones are multiple, conspicuous, isolated lesions surrounded by retinal vasculature or retina with capillary formation. In this study, the capillary-free zones could be discriminated in fundus photographs obtained by a RetCam.
All infants underwent laser treatment within 24 hours after the diagnosis of APROP. The mean post-conceptual age at the time of the first laser treatment was 35.2 weeks (range, 33 to 39.2 weeks), and second laser treatments were performed in two cases at 37.2 and 38.4 weeks post-conceptual age. After laser treatment, follow-up examinations were performed at an average of 25.2 months to evaluate anatomic and visual outcomes with complications, as summarized in Table 3. Among the 12 eyes, normal formation of the posterior pole was noted in 7 (58.3%) eyes, which were classified as anatomically-favorable outcomes. However, in the anatomically-unfavorable group, 4 eyes (33.3%) eventually progressed to tractional retinal detachment, and 1 eye (8.3%) developed retinal fold-dragging macula.
In infants with anatomically-favorable outcomes, the short- and long-term clinical courses are demonstrated in Figs. 2 and 3, respectively. During the short-term course, the areas of capillary-free zones in vascularized retina revealed late capillary filling. In Fig. 2A, 10 days after laser treatment, a broad capillary-free zone was noted in the vascularized retina. At 2 weeks after treatment, the area was slightly decreased (Fig. 2B). The gradual shrinkage of the capillary-free zones was demonstrated concurrently with capillary filling. Finally, 5 weeks after treatment, the capillary-free zones in vascularized retina resolved. The long-term follow-up examination of another patient 15 months after laser treatment revealed normal formation of the posterior pole with insignificant fibrous membrane, including previous capillary-free zones, as demonstrated in Fig. 3.
Fix-and-follow behavior was present in both eyes and BCVA was 20 / 30 in both eyes among 2 infants with favorable outcomes, in whom the behavior or visual acuity could be examined. However, fix-and-follow behavior was absent in 3 eyes, and eccentric fix was present in 1 eye, which indicated that visual outcome was associated with anatomic outcome. Complications noted in this study included glaucoma in 2 eyes (16.7%), strabismus in 1 patient (16.7%), and fine nystagmus in 1 eye (8.3%).
While APROP has been reported to occur in the smallest, most premature infants [5-7,11,12], this study demonstrated that APROP, especially with capillary-free zones in vascularized retina, can occur in infants at more advanced gestational ages and with greater birth weights compared to previously reported APROP cases. The associated factors with APROP in our study were respiratory distress syndrome (66.7%) and oxygen therapy, previously recognized risk factors for ROP [13,14]. In addition, this report demonstrated late capillary formation in capillary-free zones in vascularized retina by serial photographic documentation. These features of APROP were first revealed in this study.
Most cases of APROP occurred in ultra-premature infants with a gestational age <26 weeks and birth weights <750 g [5-7,15]. However, all of the infants in this study had gestational ages >27 weeks and birth weights >1,000 g. Therefore, these findings suggest that APROP can occur in the absence of APROP risk factors, such as extremely low gestational age and very low birth weight [5-7]. Thus, the possibility of APROP should be considered in cases of posterior ROP, regardless of gestational age and birth weight. Accordingly, frequent follow-up examinations should be performed to identify the progression of the ROP. For the other possible explanations of the atypical clinical features, the difference in the level of care in neonatal intensive care units and ethnic differences of APROP are suggested.
Complications of APROP included glaucoma, strabismus, and nystagmus. All of the cases with glaucoma occurred in patients with retinal detachment, which was consistent with the previous result, which indicated that glaucoma can develop after laser treatment for severe ROP [16]. Other complications were not remarkable compared to previous reports [5].
Regarding the pathogenesis of APROP, previous studies have discussed the relationship between ROP and its risk factors. Vascular endothelial growth factor (VEGF) is known to be essential in the normal development of retinal vasculature [17,18]. In premature babies, insulin-like growth factor-1 (IGF-1), which exerts a permissive role in VEGF-mediated new vessel growth, is at very low levels due to insufficient production by the premature liver [17,18]. Additionally, long-term hyperoxia inhibits VEGF [19-23] and more immature retinal vessels are known to be more vulnerable to oxygen toxicity [24,25]. Therefore, prematurity and hyperoxia disturb the normal development of retinal vasculature. However, for our cases in which APROP occurred in the absence of commonly associated risk factors of APROP, such as low gestational age and low birth weight, a different mechanism could be involved in the pathogenesis. Further investigation into the mechanism of APROP and into what interrupts the formation of retinal vasculature in APROP cases with relatively long gestational periods needs to be performed.
Interestingly, all of the infants with a favorable outcome of APROP revealed normal capillary formation in vascularized retina as capillary-free zones decreased gradually after laser treatment (Fig. 2). The capillary filling was done for a short period of time, at most 5 weeks after laser treatment in this study. To understand the mechanism underlying capillary filling, the normal vascular development of the retina and factors involved in the process should be considered.
Two distinct processes have been suggested for vascularization of the retina. Vasculogenesis occurs in an early phase and is responsible for the formation of major arcades. Vasculogenesis is known to be completed by 21 weeks gestation [26], and angiogenesis subsequently proceeds and forms the remaining retinal vessels, including capillaries [12]. Angiogenesis is known to be associated with VEGF, whereas vasculogenesis is insensitive to VEGF modulation. Considering the gestational period in our cases (significantly >21 weeks), and the process mediating capillary formation, we believe that angiogenesis executed the late capillary filling identified in this study.
More specifically, as infants grow, the retina matures and the serum concentration of IGF-1 rises as production in the liver increases [17]. The developing retina with a large avascular area and multiple capillary-free zones in APROP becomes hypoxic, and thus increases the expression of VEGF [17,18]. However, because of the permissive role of IGF-1 on VEGF, excessive VEGF with an increased amount of IGF-1 can result in neovascular proliferation, rather than normal vascular formation [17,18]. However, after laser treatment, the level of VEGF decreases significantly, which prevents neovascularization [17]. In addition, the retina without capillary formation within the posterior pole might produce a suitable amount of VEGF that leads to normal capillary formation on the area by the process of angiogenesis.
Previous reports have advocated that capillary-free zones in vascularized retina should be treated with laser photocoagulation [7,9]. Accordingly, the avascular retina and capillary-free zones were treated with laser photocoagulation, except for the posterior pole in our study, which has never been studied for its efficacy. Although further investigation with a larger number of infants is required, the anatomic success (58.3% in this study after laser treatment) was comparable to that previously reported for laser-treated cases of zone I ROP (54.4%) [15]. However, the rate in another study was 81.8% [5], but an anatomically-unfavorable outcome was only defined as retinal detachment. If our outcomes were similarly defined, then the anatomical success of the current study was 66.7%, which is not significantly different from the previous rate by chi-square test.
After treatment, follow-up examinations should be performed frequently to identify normal capillary formation in capillary-free vascularized retina within the posterior pole, in addition to regression signs of ROP. However, laser treatment in avascular retina alone is potentially unsafe in APROP cases because considerable production of VEGF on wide areas without capillary formation in vascularized retina might lead to neovascularization and subsequent retinal detachment.
In conclusion, this study demonstrated the late capillary filling on capillary-free zones of vascularized retina after laser treatment. For these patients, the interaction of IGF-1 and VEGF on angiogenesis and its modification after laser treatment are considered to play a role in the underlying mechanism. To validate our findings, further studies are warranted and better understandings of the pathogenesis might lead to more appropriate treatment options and more favorable outcomes in patients with APROP.
Figures and Tables
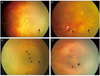 | Fig. 1Fundus photographs of patients with aggressive posterior retinopathy of prematurity revealing capillary-free zones in vascularized retina. (A,B) The infants demonstrated multiple capillary-free zones (black arrow) surrounded by retinal vasculature or retina with capillary formation. In addition, a prominent plus sign and pre-retinal hemorrhage (white arrow) were revealed. (C,D) A more highly magnified view revealing a capillary free-zone (black arrow) within vascularized retina in contrast to the area with capillary formation denoted by an arrowhead. |
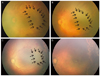 | Fig. 2Short-term course of a capillary-free zone in patient 2, who had a favorable outcome. (A-D) Serial follow-up after laser treatment (A, 10 days; B, 2 weeks; C, 4 weeks; and D, 5 weeks after the laser treatment). The capillary-free zone (bordered by black arrows) and pre-retinal hemorrhage gradually decreased after the laser treatment and resolved as shown in (D) together with regression of the plus sign. |
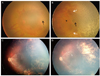 | Fig. 3Long-term clinical course of capillary-free zones demonstrated in the right eye of patient 5 (A) before laser treatment and (B) on the day of laser treatment (post-treatment). Black arrows denote capillary-free zones in vascularized retinas, in contrast to retinas with capillary formation (arrowhead). White arrows demonstrate retinal vessels, and thus the space enclosed by them indicates a vascularized zone. (C) Seven months later, capillary formation was achieved but a focal fibrous membrane was noted along the superior arcade and at the last visit, at 15 months after laser treatment, RetCam image in (D) shows a stable fibrous membrane without tractional retinal detachment, otherwise the fundus is normal. |
Table 1
Funduscopic findings of infants with aggressive posterior retinopathy of prematurity capillary-free zones at the time of diagnosis

References
1. International Committee for the Classification of Retinopathy of Prematurity. The International Classification of Retinopathy of Prematurity revisited. Arch Ophthalmol. 2005. 123:991–999.
2. Early Treatment for Retinopathy of Prematurity Cooperative Group. Revised indications for the treatment of retinopathy of prematurity: results of the early treatment for retinopathy of prematurity randomized trial. Arch Ophthalmol. 2003. 121:1684–1694.
3. Morizane H. Initial sign and clinical course of the most severe form of acute proliferative retrolental fibroplasia (type II). Nihon Ganka Gakkai Zasshi. 1976. 80:54–61.
4. Nissenkorn I, Kremer I, Gilad E, et al. 'Rush' type retinopathy of prematurity: report of three cases. Br J Ophthalmol. 1987. 71:559–562.
5. Drenser KA, Trese MT, Capone A Jr. Aggressive posterior retinopathy of prematurity. Retina. 2010. 30:4 Suppl. S37–S40.
6. Vinekar A, Trese MT, Capone A Jr. Photographic Screening for Retinopathy of Prematurity (PHOTO-ROP) Cooperative Group. Evolution of retinal detachment in posterior retinopathy of prematurity: impact on treatment approach. Am J Ophthalmol. 2008. 145:548–555.
7. Yokoi T, Hiraoka M, Miyamoto M, et al. Vascular abnormalities in aggressive posterior retinopathy of prematurity detected by fluorescein angiography. Ophthalmology. 2009. 116:1377–1382.
8. Khwarg SI, Yu HG, Yu YS. The outcome of cryotherapy for retinopathy of prematurity (ROP) according to ROP location. Korean J Ophthalmol. 1996. 10:92–96.
9. Schulenburg WE, Tsanaktsidis G. Variations in the morphology of retinopathy of prematurity in extremely low birthweight infants. Br J Ophthalmol. 2004. 88:1500–1503.
10. Section on Ophthalmology American Academy of Pediatrics. American Academy of Ophthalmology. American Association for Pediatric Ophthalmology and Strabismus. Screening examination of premature infants for retinopathy of prematurity. Pediatrics. 2006. 117:572–576.
11. Azuma N, Ishikawa K, Hama Y, et al. Early vitreous surgery for aggressive posterior retinopathy of prematurity. Am J Ophthalmol. 2006. 142:636–643.
12. Flynn JT, Chan-Ling T. Retinopathy of prematurity: two distinct mechanisms that underlie zone 1 and zone 2 disease. Am J Ophthalmol. 2006. 142:46–59.
13. Lutty GA, Chan-Ling T, Phelps DL, et al. Proceedings of the third International Symposium on Retinopathy of Prematurity: an update on ROP from the lab to the nursery (November 2003, Anaheim, California). Mol Vis. 2006. 12:532–580.
14. Mantagos IS, Vanderveen DK, Smith LE. Emerging treatments for retinopathy of prematurity. Semin Ophthalmol. 2009. 24:82–86.
15. Kychenthal A, Dorta P, Katz X. Zone I retinopathy of prematurity: clinical characteristics and treatment outcomes. Retina. 2006. 26:7 Suppl. S11–S15.
16. Trigler L, Weaver RG Jr, O'Neil JW, et al. Case series of angle-closure glaucoma after laser treatment for retinopathy of prematurity. J AAPOS. 2005. 9:17–21.
17. Chen J, Smith LE. Retinopathy of prematurity. Angiogenesis. 2007. 10:133–140.
18. Romagnoli C. Risk factors and growth factors in ROP. Early Hum Dev. 2009. 85:10 Suppl. S79–S82.
19. Alon T, Hemo I, Itin A, et al. Vascular endothelial growth factor acts as a survival factor for newly formed retinal vessels and has implications for retinopathy of prematurity. Nat Med. 1995. 1:1024–1028.
20. Ashton N, Ward B, Serpell G. Effect of oxygen on developing retinal vessels with particular reference to the problem of retrolental fibroplasia. Br J Ophthalmol. 1954. 38:397–432.
21. Claxton S, Fruttiger M. Role of arteries in oxygen induced vaso-obliteration. Exp Eye Res. 2003. 77:305–311.
22. Gu X, El-Remessy AB, Brooks SE, et al. Hyperoxia induces retinal vascular endothelial cell apoptosis through formation of peroxynitrite. Am J Physiol Cell Physiol. 2003. 285:C546–C554.
23. Pierce EA, Foley ED, Smith LE. Regulation of vascular endothelial growth factor by oxygen in a model of retinopathy of prematurity. Arch Ophthalmol. 1996. 114:1219–1228.
24. Chan-Ling T, Page MP, Gardiner T, et al. Desmin ensheathment ratio as an indicator of vessel stability: evidence in normal development and in retinopathy of prematurity. Am J Pathol. 2004. 165:1301–1313.
25. Hughes S, Chan-Ling T. Characterization of smooth muscle cell and pericyte differentiation in the rat retina in vivo. Invest Ophthalmol Vis Sci. 2004. 45:2795–2806.
26. Hughes S, Yang H, Chan-Ling T. Vascularization of the human fetal retina: roles of vasculogenesis and angiogenesis. Invest Ophthalmol Vis Sci. 2000. 41:1217–1228.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download