Abstract
Purpose
We investigated whether oxygen-induced retinopathy (OIR) results in changes in the protein expression of neuronal and inducible nitric oxide synthases (nNOS and iNOS, respectively) in rat model of OIR. In addition, we evaluated whether treatment of rats with triamcinolone acetonide (TA) prevents this response.
Methods
To promote OIR, Sprague-Dawley rats were exposed to hyperoxia from postnatal day 2 (P2) to P14. They were then returned to normoxia after P15. TA was injected into the right vitreous of P15 rats, while saline was injected into the left vitreous. At P18 the expression of nNOS and iNOS was determined using Western blotting and immunostaining techniques in retinas obtained from control rats.
Results
In P18 OIR rats, the abundance of nNOS and iNOS protein was significantly increased compared with controls. These increases were not observed in the retinas of rats treated with TA. The change in expression of nNOS and iNOS were specific to parvalbumin and glial fibrillary acidic protein-positive cells. Treatment with TA prevented the increased expression of nNOS and iNOS in all samples.
Hypoxia is one of the leading causes of retinal vascular pathology and neuronal cell damage, which are commonly observed in retinal pathologies such as retinopathy of prematurity (ROP) and diabetic retinopathy [1,2]. Oxygen-induced retinopathy (OIR) in neonatal rats, under conditions where neuronal damage is induced by hypoxic retinal damage, is a widely used animal model of ROP [3-5]. Kim et al. [6] reported that the most severe retinal injury from hyperoxic/normoxic injury appears in OIR rat retinas at postnatal day 18 (P18) and 4 days after relative hypoxia (from P2 to P14).
Under certain pathological conditions, expression of neuronal and inducible nitric oxide synthase (nNOS and iNOS, respectively) might be enhanced, resulting in neurotoxicity and subsequent neurodegeneration [7,8]. Kaur et al. [9] and Kaur et al. [10] suggested that increased vascular endothelial growth factor (VEGF) and nitric oxide (NO) production in hypoxia results in increased vascular permeability and degeneration of neural cells. The mechanism leading to excessive nNOS activation is thought to be mediated by hyperactivation of glutamate receptors, as well as possible mitochondrial dysfunction and cell death [11]. In addition, NO is postulated as a key neurotoxic factor of ROP and an increase in NO concentration in retina induces cytotoxic effects [12-15].
Triamcinolone acetonide (TA) is a corticosteroid with anti-inflammatory and anti-angiogenic effects that has been reported to influence cellular events in vascular and neural tissues through the activation of cytokines [9,10,16-18]. Various reports have suggested that TA may have a neuroprotective effect in retinal disorders and may be involved in neuroprotection of neuronal and glial cells [19,20]. In a model of OIR, Hartnett et al. [21] suggested that TA reduces neovascularization and capillary density, while Park et al. [22] reported that TA protects retinal neurons damaged by relative hypoxia from decreased decorin levels.
In this study, we describe the effect of TA treatment on the expression of nNOS and iNOS in the retinas of OIR rats.
Pregnant Sprague-Dawley (SD) rats were purchased from KOATEC (Pyeongtaek, Korea). Rats were individually housed under alternating 12-hour light/dark cycles and given rat chow and water, in accordance Use Committee of Gyeongsang National University.
For promotion of OIR, five new born SD rat pups were exposed to hyperoxia (80 ± 1.3% O2) from P2 to P14 (with 2 hr/day in room air), and then returned to normal conditions (room air, 21 ± 1.5% O2) from P14 to P18, as previously described [6]. Five control rats were maintained in room air. All rats were sacrificed at P18 to investigate changes in OIR rat retinas compared with normal retinas.
All rats were anesthetized at P15 to assess the effect of TA (Hanall Co., Seoul, Korea) on the retinas of OIR and control rats. Using a 30-gauge needle, a TA solution (2 µL, 40 mg/mL stock solution in saline) was injected under an operating microscope through the pars plana into the right vitreous, and a saline solution (2 µL) was injected into the left vitreous.
Total protein extraction and Western blot analysis was performed as previously described [6]. Retinal protein isolated from five OIR and five control rats at P18 with TA or saline treatment were subjected to 10% sodium dodecyl sulphate polyacrylamide gel electrophoresis and transferred to a nitrocellulose membrane. The blots were incubated in mouse monoclonal anti-nNOS antibody (1 : 1,000 dilution; BD Biosciences, San Jose, CA, USA) and in rabbit polyclonal anti-iNOS antibodies (1 : 500 dilution; Abcam, Cambridge, UK), followed by incubation with horseradish peroxidase-conjugated rabbit anti-mouse and goat anti-rabbit IgGs (Pierce, Rockford, IL, USA). Specific proteins were visualized using an enhanced chemiluminescent kit according to the manufacturer's instructions (Amersham Bioscience, Piscataway, NJ, USA). Blots were re-probed with an α-tubulin antibody (1 : 20,000 dilution; Sigma, St. Louis, MO, USA) as a control for loading. Changes in expression of retinal nNOS and iNOS protein were measured in five independent tests.
Immunohistochemistry was performed on 12-µm retinal frozen sections from P18 OIR and control rats, as previously described [6]. Sections were blocked in 1.5% normal goat serum for 30 min at room temperature (RT), incubated with primary nNOS and iNOS antibodies (1 : 200 dilution, respectively) for 2 hours at 4℃, washed three times with 10 mmol/L Tris-buffered saline, and incubated with biotinylated anti-rabbit IgG antibody (1 : 200; Santa Cruz Biotechnology, Santa Cruz, CA, USA) in Tris buffer for 1 hour at RT. Following an additional wash with Tris buffer, sections were incubated with avidin-biotinylated horseradish peroxidase complex (ABC; Vector Laboratories Inc., Burlingame, CA, USA) and treated with 0.025% 3,3'-diaminobenzidine tetrahydrochloride (Sigma) in 0.003% H2O2.
Double immunofluorescence was performed in the following manner. After washing with Tris buffer, the sections were then incubated at RT with a cocktail of two primary antibodies, anti-nNOS and anti-parvalbumin as markers for amacrine cells, and iNOS and GFAP as markers for astrocytes. For immunofluorescent staining, Alexa Fluor-488 or -594 goat anti-rabbit or -mouse IgG were employed as secondary antibodies (1 : 200; Pierce; Invitrogen, Carlsbad, CA, USA). After several washes with Tris buffer, the sections were mounted using ProLong Gold antifade reagent (Invitrogen).
Retinal images were captured at a distance of approximately 0.8 to 1.0 mm from the optic nerve head using an IX2-DSU disk scanning confocal microscope (Olympus, Wendenstrasse, Hamburg, Germany). The number of cells that had a immunoreactive nNOS signal was quantified in the ganglion cell layer (GCL) and inner nuclear layer (INL) in whole fields containing five different retinas of saline or TA treated control and OIR rats group. Each experiment was independently performed five times using five different retinal samples for each group.
Densitometry measurements were processed using SigmaGel ver. 1.0 (Jandel Scientific, Erkrath, Germany) and SigmaPlot ver. 4.0 (SPSS Inc., Chicago, IL, USA). The significance of inter-group differences was evaluated after α-tubulin normalization in each group by Kruskal Wallis H-test and Mann-Whitney U-testing (SPSS, SPSS Inc.). Data are presented as mean ± SE. Differences were considered significant at p < 0.05.
nNOS protein appeared as a single band of approximately 155 kDa when subjected to electrophoresis (Fig. 1A). We found increased retinal levels of nNOS protein at P18 in saline injected OIR rats (2.12-fold, p = 0.00134, n = 5) (Fig. 1B) compared with saline injected control rats. Once again, treatment with TA prevented the increase in retinal nNOS protein in OIR rats compared with saline injected control rats (1.00-fold, p = 0.625, n = 5) (Fig. 1B). The difference in expression of nNOS protein expression between TA and saline treated P18 rats was significant (p = 0.00057) (Fig. 1B).
iNOS protein appeared as a single band of approximately 130 kDa (Fig. 1C). We found increased retinal levels of iNOS protein at P18 in saline injected OIR rats (2.99-fold, p = 0.0027, n = 5) (Fig. 1D) compared with saline injected control rats. Treatment with TA prevented increased abundance of retinal iNOS protein in OIR rats compared with saline injected control rats (1.58-fold, p = 0.1371, n=5) (Fig. 1D). In addition, the difference in expression of iNOS protein in P18 OIR rats with or without TA treatment was significant (p = 0.011) (Fig. 1D).
Retinal nNOS immunoreacitvity was observed in neuronal cells, especially amacrine cells, in the GCL and INL of retinas from saline injected control rats (Fig. 2A). Increased nNOS immunoreactivity was more prevalent in the GCL and INL of retinas of saline injected OIR rats compared with saline injected control rats at P18 (Fig. 2B). Treatment with TA in OIR rats led to nNOS immunoreactivity similar to that observed in the retinas of saline injected control rats (Fig. 2C). Double staining revealed an overlap of nNOS signal (green) and the parvalbumin signal (red) in the GCL and INL of retinas of saline injected OIR rats (Fig. 2D). The nNOS-immunoreactive cells in the inner retina were determined to be parvalbumin-positive amacrine cells. The number of nNOS-positive amacrine cells in saline injected P18 OIR rats was significantly increased versus saline injected control rats (2.05-fold, p = 0.00001, n = 5) (Fig. 3A). The increase of nNOS-positive amacrine cells in P18 OIR rats was blocked by treatment of TA compared with saline injected control rats (1.43-fold, p = 0.0088, n = 5) (Fig. 3A). Furthermore, the difference in the number of nNOS-positive amacrine cells in P18 OIR rats between groups with and without treatment was significant (p = 0.00385) (Fig. 3A).
Weak iNOS expression was detected in the nerve fiber layer (NFL) of retinas from saline injected control rats (Fig. 2D). Increased iNOS immunoreactivity was observed in the NFL of saline injected OIR rats compared with saline injected control rats at P18 (Fig. 2F). Treatment with TA prevented the increase of nNOS immunoreactivity in OIR rats. Expression levels were similar to that seen in the retinas of saline injected control rats (Fig. 2G). Double immunofluorescent staining revealed an overlap of the iNOS signal (red) and the GFAP signal (green) in the NFL of saline injected OIR rats (Fig. 2H). The iNOS-immunoreactive cells observed in the inner retina were determined to be GFAP-positive astrocytes. The iNOS immunoreactivity in saline injected P18 OIR rats was significantly increased versus saline injected control rats (2.57-fold, p = 0.0012, n = 5) (Fig. 3B). The increase of iNOS expression in P18 OIR rats was blocked by treatment of TA compared with saline injected control rats (1.22-fold, p = 0.1173, n = 5) (Fig. 3B). In addition, the difference in iNOS expression in P18 OIR rats between rats with and without TA treatment was significant (p = 0.00035) (Fig. 3B).
In this study, we examined the expression of nNOS and iNOS in the retinas of OIR rats. In previous studies, the expression of nNOS was found to increase in rat models of chronic glaucoma, diabetic retinopathy, and optic nerve transaction [16,23-25]. Other reports have suggested that the protein expression of eNOS and nNOS is increased in the OIR model, while the expression of iNOS is decreased [26]. This discrepancy is thought due to differences of experimental OIR models, especially the distribution of hyperoxia and length of normoxia period. In the present study, significant increases were observed for the expression of nNOS and iNOS in the OIR rat retina compared with control rats. Thus, under hypoxic conditions, nNOS and iNOS activation might be increased, leading to cellular changes that may play a role in neurodegeneration.
The developing eye and visual system have been reported to be susceptible to hypoxic-ischemic damage [27]. Oxygen deprivation induces overproduction of NO, an excess of which is toxic to cells [28,29]. Given that, at the retina, neurotoxicity induced by hypoxia is related to alterations in nitric oxide regulation [12], it may be suggested that the increased expression of iNOS and nNOS in the OIR retinas observed in our study might be responsible for neuronal damage.
Recently, Rey-Funes et al. [15] reported that hypothermia is protective against damage of the retina induced by perinatal asphyxia, which was due to inhibition of alterations of both NOS isoforms and nitration of proteins. Several strategies such as the use of antioxidant and dexamethasone have been tried in order to prevent hypoxia-ischemic injury [30,31]. Previously, we found retinal vascular pathologic changes and gene expression changes in OIR rats at P18 and demonstrated suppression of these changes via interruption of VEGF activation through exposure to TA [6,31,32]. In the present study, TA blocked an increase of iNOS and nNOS expression, suggesting that TA may be protective against damage of retina in OIR rat retinas. However, the mechanism by which treatment with TA prevents increased nNOS and iNOS expression has not been defined. Additional experiments will be required to determine whether TA treatment may affect neuronal cell damage and regeneration through the activation/inhibition of various cytokines in the hypoxic retina.
We analyzed a number of nNOS-positive cells in the INL and the GCL. The increase in the number of nNOS-positive amacrine cells in the INL and the GCL was determined in the retinas of OIR rats. In the retinas of normal rats, nNOS immunoreactivity was localized to certain populations of amacrine cells found in the INL and in a small group of neurons localized in the GCL [33]. These nNOS-containing neurons exhibited their own basal expression levels of nNOS in the retinal layers, and thus these neurons might be expected to react differentially in response to retinal ischemia [34]. Oh et al. [35] observed that nNOS-expressed neurons in normal rats co-localizes with gamma aminobutyric acid (GABA), suggesting that an increase of nNOS-expressing amacrine cells might be accompanied by dysfunction of GABA metabolism in diabetic retina. In addition, Rey-Funes et al. [15] demonstrated colocalizaion of nNOS and tyrosine hydroxylase (TH) in the cytoplasm of a subpopulation of ganglion and amacrine cells of perinatal asphyxia model rats, which could be prevented by hypothermia. However, in our study, nNOS-positive cells were co-localized with parvalbumin but not in GABA, choline acetyltransferase or TH (data not shown) expressing cells, future studies will be needed to determine the reason for this discrepancy. Otherwise, the present study using double immunofluorescence labeling with GFAP (a marker for astrocytes) and iNOS showed complete colocalization in the retina of OIR rats as reported previously [15,35].
In conclusion, we have shown that hypoxia leads to upregulation of nNOS and iNOS in the OIR rat retinas, which can be prevented by treatment with TA. Further studies should be carried out to elucidate the direct responsibility of NO and NOS activity in OIR rats, including identifying the molecular mechanism of TA-induced alterations of NOS systems.
Figures and Tables
Fig. 1
(A) Western blot of retinal neuronal nitric oxide synthase (nNOS) protein in postnatal day 18 (P18) oxygen-induced retinopathy (OIR) rats versus control rats. A single band of approximately 155 kDa was detected in total retina protein extracts of P18 OIR rats and control rats. Levels of nNOS protein in the retina increased in saline treated P18 OIR rats versus saline treated control rats, and this increase was prevented by treatment with triamcinolone acetonide (TA). (B) Bar graphs representing retinal nNOS protein expression showing significant changes between saline treated P18 OIR rats and saline treated control rats (**p < 0.001). The increase in expression of nNOS protein is markedly decreased in P18 OIR treated with TA (†p < 0.05) when compared with saline treated controls. (C) Western blot for inducible nitric oxide synthase (iNOS) protein in the retinas of P18 OIR rats versus control rats. A single band of approximately 130 kDa was detected in total retina protein extracts of P18 OIR rats and control rats. Levels of retinal iNOS protein increased in saline treated P18 OIR rats versus saline treated control rats. This increase was blocked by treatment with TA. (D) Bar graphs representing retinal iNOS protein expression showing significant changes between saline treated P18 OIR rats and saline treated control rats (**p < 0.001). A significant reduction in retinal iNOS protein was observed for rats treated with TA (†p < 0.05). TAA = triamcinolone acetonide; CTL = control.
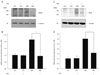
Fig. 2
Photomicrographs of retinal coronal sections showing neuronal nitric oxide synthase (nNOS) and inducible nitric oxide synthase (iNOS) immunoreactivity. (A) nNOS-immunoreactive cells were detected in the ganglion cell layer (GCL, presented as small arrow) and inner nuclear layer (INL, presented as large arrow) in saline treated control rats. (B) nNOS immunoreactivity was increased in the GCL and INL in saline treated postnatal day 18 (P18) oxygen-induced retinopathy (OIR) rats. (C) Treatment with triamcinolone acetonide (TA) restored nNOS immunoreactivity in the GCL and INL to levels similar to that of saline treated control rats. Scale bar = 25 µm. (D) Yellow-signals (presented as white arrow) co-stained with parvalbumin (red) and nNOS (green) were observed in the INL of saline treated OIR rats in double immunofluorescent staining. (E) Weak iNOS immunoreactivity was detected in the nerve fiber layer (NFL) in saline treated control rats. (F) iNOS immunoreactivity was markedly increased in the NFL (presented as arrowhead) in saline treated P18 OIR rats. (G) Treatment with TA prevented the increase in iNOS immunoreactivity in the retinas of P18 OIR rats. (H) Yellow-signals (presented as white arrowhead) co-stained with iNOS (red) and GFAP (green) were observed in the NFL of saline treated OIR rats in double immunofluorescent staining.

Fig. 3
(A) Graphical representation of the changes in magnitude of staining for neuronal nitric oxide synthase (nNOS)-positive amacrine cells in postnatal day 18 (P18) oxygen-induced retinopathy (OIR) rats versus control rats. nNOS-positive amacrine cells in saline treated P18 OIR rats were increased versus saline treated control rats (**p < 0.001). Treatment with triamcinolone acetonide (TA) prevented an increase of nNOS-positive cells (†p < 0.05). (B) Changes in the magnitude of staining for inducible nitric oxide synthase (iNOS) immunoreactivity in the nerve fiber layer of saline treated P18 OIR rats versus control rats. iNOS immunoreactivity in saline treated P18 OIR rats was versus saline treated control rats (**p < 0.001). Treatment with TA prevented the increase of iNOS immunoreactivity (†p < 0.05). TAA = triamcinolone acetonide; CTL = control.

Acknowledgements
This work was supported by grant no. 2009-0068732 from the Basic Research Program of the Korea Science & Engineering Foundation. In addition, this study was partially supported by the BK21 Program and by the MRC program of KRF (R13-2005-012-01001-1). We would like to thank Ae-Jin Gu for her technical assistance.
References
1. Brafman A, Mett I, Shafir M, et al. Inhibition of oxygen-induced retinopathy in RTP801-deficient mice. Invest Ophthalmol Vis Sci. 2004. 45:3796–3805.
2. Hammes HP, Lin J, Renner O, et al. Pericytes and the pathogenesis of diabetic retinopathy. Diabetes. 2002. 51:3107–3112.
3. Inatani M, Tanihara H, Honjo M, et al. Expression of proteoglycan decorin in neural retina. Invest Ophthalmol Vis Sci. 1999. 40:1783–1791.
4. Ishida S, Usui T, Yamashiro K, et al. VEGF164-mediated inflammation is required for pathological, but not physiological, ischemia-induced retinal neovascularization. J Exp Med. 2003. 198:483–489.
5. Downie LE, Pianta MJ, Vingrys AJ, et al. Neuronal and glial cell changes are determined by retinal vascularization in retinopathy of prematurity. J Comp Neurol. 2007. 504:404–417.
6. Kim YH, Chung IY, Choi MY, et al. Triamcinolone suppresses retinal vascular pathology via a potent interruption of proinflammatory signal-regulated activation of VEGF during a relative hypoxia. Neurobiol Dis. 2007. 26:569–576.
7. Dawson VL, Dawson TM. Nitric oxide neurotoxicity. J Chem Neuroanat. 1996. 10:179–190.
8. Dawson VL, Dawson TM, London ED, et al. Nitric oxide mediates glutamate neurotoxicity in primary cortical cultures. Proc Natl Acad Sci U S A. 1991. 88:6368–6371.
9. Kaur C, Sivakumar V, Foulds WS, et al. Cellular and vascular changes in the retina of neonatal rats after an acute exposure to hypoxia. Invest Ophthalmol Vis Sci. 2009. 50:5364–5374.
10. Kaur C, Sivakumar V, Foulds WS. Early response of neurons and glial cells to hypoxia in the retina. Invest Ophthalmol Vis Sci. 2006. 47:1126–1141.
11. Almeida A, Heales SJ, Bolanos JP, Medina JM. Glutamate neurotoxicity is associated with nitric oxide-mediated mitochondrial dysfunction and glutathione depletion. Brain Res. 1998. 790:209–216.
12. Osborne NN, Casson RJ, Wood JP, et al. Retinal ischemia: mechanisms of damage and potential therapeutic strategies. Prog Retin Eye Res. 2004. 23:91–147.
13. Chemtob S, Hardy P, Abran D, et al. Peroxide-cyclooxygenase interactions in postasphyxial changes in retinal and choroidal hemodynamics. J Appl Physiol. 1995. 78:2039–2046.
14. Hardy P, Dumont I, Bhattacharya M, et al. Oxidants, nitric oxide and prostanoids in the developing ocular vasculature: a basis for ischemic retinopathy. Cardiovasc Res. 2000. 47:489–509.
15. Rey-Funes M, Ibarra ME, Dorfman VB, et al. Hypothermia prevents nitric oxide system changes in retina induced by severe perinatal asphyxia. J Neurosci Res. 2011. 89:729–743.
16. Lee EJ, Kim KY, Gu TH, et al. Neuronal nitric oxide synthase is expressed in the axotomized ganglion cells of the rat retina. Brain Res. 2003. 986:174–180.
17. Abraham IM, Harkany T, Horvath KM, Luiten PG. Action of glucocorticoids on survival of nerve cells: promoting neurodegeneration or neuroprotection? J Neuroendocrinol. 2001. 13:749–760.
18. Jonas JB, Kreissig I, Degenring R. Intravitreal triamcinolone acetonide for treatment of intraocular proliferative, exudative, and neovascular diseases. Prog Retin Eye Res. 2005. 24:587–611.
19. Ozdek SC, Aydin B, Gurelik G, et al. Effects of intravitreal triamcinolone injection on macular edema and visual prognosis in central retinal vein occlusion. Int Ophthalmol. 2005. 26:27–34.
20. Psarra AM, Bochaton-Piallat ML, Gabbiani G, et al. Mitochondrial localization of glucocortocoid receptor in glial (Müller) cells in the salamander retina. Glia. 2003. 41:38–49.
21. Hartnett ME, Martiniuk DJ, Saito Y, et al. Triamcinolone reduces neovascularization, capillary density and IGF-1 receptor phosphorylation in a model of oxygen-induced retinopathy. Invest Ophthalmol Vis Sci. 2006. 47:4975–4982.
22. Park YJ, Kim YH, Choi WS, et al. Treatment with triamcinolone acetonide prevents decreased retinal levels of decorin in a rat model of oxygen-induced retinopathy. Curr Eye Res. 2010. 35:657–663.
23. Park JW, Park SJ, Park SH, et al. Up-regulated expression of neuronal nitric oxide synthase in experimental diabetic retina. Neurobiol Dis. 2006. 21:43–49.
24. Park SH, Kim JH, Kim YH, Park CK. Expression of neuronal nitric oxide synthase in the retina of a rat model of chronic glaucoma. Vision Res. 2007. 47:2732–2740.
25. Leal EC, Manivannan A, Hosoya K, et al. Inducible nitric oxide synthase isoform is a key mediator of leukostasis and blood-retinal barrier breakdown in diabetic retinopathy. Invest Ophthalmol Vis Sci. 2007. 48:5257–5265.
26. Kim WT, Suh ES. Retinal protective effects of resveratrol via modulation of nitric oxide synthase on oxygen-induced retinopathy. Korean J Ophthalmol. 2010. 24:108–118.
27. Jacobson LK, Dutton GN. Periventricular leukomalacia: an important cause of visual and ocular motility dysfunction in children. Surv Ophthalmol. 2000. 45:1–13.
28. Bredt DS, Snyder SH. Nitric oxide, a novel neuronal messenger. Neuron. 1992. 8:3–11.
29. Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991. 43:109–142.
30. Raju TN, Langenberg P, Bhutani V, Quinn GE. Vitamin E prophylaxis to reduce retinopathy of prematurity: a reappraisal of published trials. J Pediatr. 1997. 131:844–850.
31. Rotschild T, Nandgaonkar BN, Yu K, Higgins RD. Dexamethasone reduces oxygen induced retinopathy in a mouse model. Pediatr Res. 1999. 46:94–100.
32. Wenzel A, Grimm C, Seeliger MW, et al. Prevention of photoreceptor apoptosis by activation of the glucocorticoid receptor. Invest Ophthalmol Vis Sci. 2001. 42:1653–1659.
33. Gwon JS, Ju WK, Park SJ, et al. The regulatory expression of neuronal nitric oxide synthase in the ischemic rat retina. Neuroreport. 2001. 12:3385–3389.
34. Bolanos JP, Almeida A. Roles of nitric oxide in brain hypoxia-ischemia. Biochim Biophys Acta. 1999. 1411:415–436.
35. Oh SJ, Kim IB, Lee MY, et al. NOS-like immunoreactive neurons express GABA-like immunoreactivity in rabbit and rat retinae. Exp Brain Res. 1998. 120:109–113.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download