Abstract
Purpose
To evaluate whether a combination of penetrating keratoplasty (PKP) or pars plana vitrectomy (PPV) and Ahmed glaucoma valve (AGV) implantation affords a level of success similar to that of AGV implantation alone.
Methods
Eighteen eyes underwent simultaneous PPV and AGV, 14 eyes with PKP and AGV and 30 eyes with AGV implantation alone were evaluated. Success was defined as attainment of an intraocular pressure (IOP) >5 and <22 mmHg, with or without use of anti-glaucoma medication. Kaplan-Meier survival analysis was performed to compare cumulative survival between the combined surgery groups and the AGV implantation-alone group. Cox proportional hazard regression analysis was conducted to identify factors predictive of success in each of the three groups.
Results
Mean (±standard deviation) preoperative IOP was 30.2 ± 10.2 mmHg in the PKP + AGV, 35.2 ± 9.8 mmHg in the PPV + AGV, and 36.2 ± 10.1 mmHg in the AGV implantation-alone group. The cumulative success rate at 18 months was 66.9%, 73.2%, and 70.8% in the three groups, respectively. Neither combined surgery group differed significantly in terms of cumulative success rate compared with the AGV implantation-alone group (p = 0.556, p = 0.487, respectively). The mean number of preoperative anti-glaucoma medications prescribed was significantly associated with success in the PKP + AGV implantation group (hazard ratio, 2.942; p = 0.024).
Conclusions
Either PKP or PPV performed in conjunction with AGV implantation afforded similar success rates compared to patients treated with AGV implantation alone. Therefore, in patients with refractory glaucoma who have underlying corneal or retinal pathology requiring treatment with PKP or PPV, AGV implantation can be performed simultaneously.
In patients with uncontrolled intraocular pressure (IOP) despite prescription of maximum tolerated medical therapy (MTMT), surgical management such as trabeculectomy or glaucoma drainage device (GDD) implantation should be considered. GDD implantation is usually preferred when conventional trabeculectomy has already failed or is likely to fail.
Among such refractory patients, some need other forms of simultaneous intraocular surgery. For example, visually significant corneal opacity can present with refractory glaucoma that is not responsive to medical treatment. Patients with iridocorneal endothelial syndrome, herpetic keratouveitis, trauma, aphakic or pseudophakic bullous keratopathy, or congenital glaucoma may need penetrating keratoplasty (PKP). Some patients also present with vitreous hemorrhage and/or retinal detachment, together with uncontrolled IOP; such patients require simultaneous pars plana vitrectomy (PPV) and an IOP-lowering procedure.
Simultaneous surgery has both advantages and disadvantages. The principal value of simultaneous surgery is avoidance of multiple procedures. If PKP or PPV is performed on patients with uncontrolled IOP without the use of a simultaneous IOP-lowering procedure, IOP elevation may be aggravated, and an immediate second operation may be required before the initial surgery site has healed. If an IOP-lowering procedure such as GDD implantation alone is initially performed, subsequent PKP or PPV may aggravate the wound associated with implantation, thereby worsening the outcome of the earlier procedure.
However, simultaneous surgery may require a longer surgical time, which may in turn be negatively associated with surgical outcome and the prevalence of postoperative complications. In the current study, we evaluated the outcomes of patients undergoing intraocular surgery (PPV or PKP) with concurrent Ahmed glaucoma valve (AGV) implantation. We compared the outcomes to those of patients receiving AGV implantation alone.
Eighteen eyes of 18 consecutive patients who underwent simultaneous PPV and AGV implantation and 14 eyes of 13 consecutive patients who underwent simultaneous PKP and AGV implantation at the glaucoma clinic of the Asan Medical Center (Seoul, Korea) and who met the inclusion criteria were included in the present study. All surgery was performed between March 2008 and June 2010. Of the 143 eyes that underwent AGV implantation alone over the same period, 30 were randomly chosen as controls. All AGV implantation was performed by a single surgeon (KRS). All included patients were followed-up postoperatively for at least 1 year.
The surgical procedure was carried out using a fornix-based conjunctival flap constructed at the superotemporal or inferotemporal area. The inferotemporal area was chosen for AGV implantation in eyes for which silicone oil filling during PPV was planned. A limbus-based half-thickness scleral flap (5 mm circumferentially × 7 mm radially) was then prepared. AGV patency was tested, and the valve was placed under Tenon's capsule, 8-10 mm posterior from the superotemporal or inferotemporal limbus, and sutured using 9-0 nylon to ensure fixation. The tube portion of the AGV was temporarily placed under the conjunctival flap. Either PPV or PKP was performed. After completion of the chosen procedure, AGV implantation was resumed. Paracentesis was performed at the temporal sclerocorneal junction, and viscoelastics were injected to prevent a sudden drop in IOP during entry of the proximal tube into the anterior chamber. Chamber entry was initiated under the scleral flap. Paracentesis was performed under this flap using a 23-gauge needle; the procedure was conducted 2 mm posterior to the limbus, parallel to the iris plane. The proximal portion of the tube was sized to ensure that the tube length within the anterior chamber, measured from the limbus, was approximately 2 mm. The tube was inserted, in the bevel-up position, into the anterior chamber. The scleral flap was next sutured with 9-0 nylon, and a watertight conjunctival closure was performed. Topical corticosteroid, cycloplegics, and an antibiotic were prescribed for approximately 1 month postoperatively, depending on the condition of the eye. Follow-up examinations were performed on postoperative day 1, and at 1 week, 1 month, 3 months, 6 months, 9 months, and 1 year. If necessary, additional visits were scheduled. The study was approved by the institutional review board of the Asan Medical Center and followed the principles of the Declaration of Helsinki.
At each visit, IOP was measured by Goldmann applanation tonometry, best-corrected visual acuity was assessed, use of anti-glaucoma medication was noted, and complications were recorded. Overall success was defined as attainment of an IOP >5 and <22 mmHg with or without use of anti-glaucoma medication, without additional glaucoma surgery or removal of the AGV, and without development of any serious complication. Performance of further surgery, such as re-PKP or re-PPV, that did not seek to treat glaucoma was not considered failure. Kaplan-Meier survival analysis with log-rank testing was performed to compare cumulative success rates between each combined surgery group and the AGV implantation-alone group. Cox proportional hazard regression analysis was conducted to determine factors associated with success in each group. Putative factors included baseline age, preoperative IOP level, a history of previous intraocular surgery, and the number of anti-glaucoma medications used preoperatively. A p-value less than 0.05 was considered significant. All statistical analysis was performed using SPSS version 15.0 (SPSS Inc., Chicago, IL, USA) and MedCalc version 11.3.6.0 (MedCalc, Mariakerke, Belgium).
Mean age, preoperative IOP levels, and preoperative diagnoses in each of the three groups are summarized in Table 1. Mean (±standard deviation) preoperative IOP was 30.2 ± 10.2 mmHg in the PKP + AGV implantation group, 35.2 ± 9.8 mmHg in the PPV + AGV implantation group, and 36.2 ± 10.1 mmHg in the AGV implantation-alone group. The most common preoperative diagnosis was pseudophakic bullous keratopathy with secondary angle closure glaucoma in the PKP + AGV implantation group (4 eyes), diabetic vitreous hemorrhage with neovascular glaucoma (NVG) in the PPV + AGV implantation group (5 eyes), and NVG in the AGV implantation-alone group (18 eyes).
The mean postoperative IOP values and the numbers of anti-glaucoma medications used are shown in Table 2. In the PKP + AGV implantation group, the mean postoperative IOP value ranged between 13.6 to 19.6 mmHg. The mean postoperative IOP values at each visit did not significantly differ from those of the AGV implantation-alone group except at 1 week postoperatively (16.9 vs. 11.9 mmHg, p = 0.006). The mean IOP on postoperative day 1 was significantly higher in the PPV + AGV implantation group than in the AGV implantation-alone group (20.6 vs. 13.8 mmHg, p = 0.004). However, IOP values at all other visits did not differ significantly between the two groups. The mean number of anti-glaucoma medications used postoperatively did not differ among the combined surgery groups and the AGV implantation-alone group at any visit (Table 2).
Three of 14 eyes (21.4%) in the PKP + AGV implantation group failed to respond to treatment at 1 year postoperatively because of persistent hypotony (1 eye), AGV exposure (1 eye), or development of uncontrolled IOP with MTMT requiring additional AGV implantation (1 eye). Three of 18 eyes (16.7%) in the PPV + AGV implantation group failed to respond to treatment at 1 year postoperatively because of persistent hypotony (2 eyes) or development of uncontrolled IOP with MTMT requiring additional AGV implantation (1 eye). Six of 30 eyes (20%) in the AGV implantation-alone group failed to respond to treatment during the same follow-up period. Of these 6 eyes, three showed persistent hypotony, 2 AGV implant exposure, and 1 required repeat AGV implantation because of uncontrolled IOP.
The cumulative success rate at 18 months postoperatively was 66.9% in the PKP+AGV implantation group, 73.2% in the PPV + AGV implantation group, and 70.8% in the AGV implantation-alone group. The cumulative success rates did not differ significantly when either combined surgery group was compared with the AGV implantation-alone group (log rank test; p = 0.556, p = 0.487, respectively) (Fig. 1).
Among the various putative risk factors, the mean number of preoperative anti-glaucoma medications used was significantly associated with success in the PKP + AGV implantation group (hazard ratio, 2.942; p = 0.024). No factor analyzed was significantly associated with success in the PPV + AGV implantation or the AGV implantation-alone groups (Table 3).
Among the 14 eyes in the PKP + AGV implantation group, 3 underwent re-PKP because of corneal graft failure evident during follow-up. Among the 18 eyes in the PPV + AGV implantation group, 2 underwent re-PPV because of recurrent vitreous hemorrhage.
Overall, a combination of PKP or PPV and AGV implantation substantially reduced IOP for at least 1 year postoperatively. Mean postoperative IOP and the number of anti-glaucoma medications used were significantly lower (compared to preoperative values) at each follow-up time-point in both combined surgery groups.
The outcomes of patients in the PKP + AGV group were in line with those of earlier reports. The frequency of successful IOP control after combined PKP and GDD implantation ranges from 65% to 95% [1-6]. Coleman et al. [6] reported a 62% success rate at 20 months in a series of 12 patients when simultaneous PKP and AGV implantation were performed. Kwon et al. [5], using different types of implants, experienced a higher success rate of 82% at 3 years postoperatively.
Few reports have studied the simultaneous use of PPV and AGV implantation for concurrent treatment of retinal disease and IOP elevation. Faghihi et al. [7] reported a success rate of 72.2%, where success was defined as attainment of an IOP of 5 to 21 mmHg with or without use of antiglaucoma medication. The success rate reported in the present using combined PPV and AGV implantation was similar. A procedural difference in terms of tube entry is evident when the cited work is compared with our present report. We employed anterior chamber entry in all instances, whereas the cited authors inserted the tube into the pars plana after PPV.
In patients receiving AGV implantation alone, the cumulative success rate was 70.8% at 18 months postoperatively. Comparisons of surgical results are generally very difficult because outcomes can vary depending on factors such as heterogeneity of underlying disease; a history of previous surgery; the use of differing inclusion, exclusion, and success criteria; varying ethnicities; differences in follow-up period; and varying surgical techniques. Thus, the reported success rates of GDD placement vary considerably among different studies [8]. Minckler et al. [9] reported a 40% success rate after placement of Molteno implants with medication. Coleman et al. [10] experienced a 78% success rate employing AGVs, again with medication, whereas Lloyd et al. [11] reported a 70% success rate using Baerveldt implants with medication.
The causes of failure in all three groups were persistent hypotony and uncontrolled IOP requiring additional AGV implantation. When we assessed the risk factors for failure in each group employing Cox proportional hazards modeling, the number of anti-glaucoma medications used prior to treatment was significant in the PKP + AGV group. Intensive use of topical anti-glaucoma medication induces subclinical inflammation and metaplasia of the conjunctiva. The surgical outcome of a filtering operation is subsequently affected [12-16] and could explain the observed association between the use of a greater number of preoperative anti-glaucoma medications and surgical failure in the PKP + AGV implantation group. However, no factor analyzed was significant in the other two groups.
A combination of AGV implantation with another intraocular procedure offers several advantages compared to multiple-stage operations. However, concerns have been raised as to whether combined surgery could yield success rates similar to those seen after AGV implantation alone in terms of IOP control. PKP and PPV are the most invasive procedures used in the treatment of ocular disease. Therefore, we analyzed outcomes in patients treated with PKP or PPV who simultaneously underwent AGV implantation and compared them with those of AGV implantation-alone patients. All AGV implantation procedures were performed by a single surgeon. Thus, under similar conditions, combination of either PKP or PPV with AGV implantation afforded success rates in terms of glaucoma control similar to that of AGV implantation alone, and the complication pattern did not differ. Use of a higher number of anti-glaucoma medications was associated with surgical failure in the PKP + AGV implantation group.
The limitation of current study is that it includes a relatively small number of cases; therefore, studies with larger sample sizes are warranted. We have conclusively shown that AGV implantation combined with PKP or PPV is both safe and convenient and affords good success rates. The use of these combined procedures avoids the need for successive surgery, which inevitably induces conjunctival scarring and is associated with adverse outcomes. Therefore, in patients with refractory IOP elevation and underlying corneal or retinal pathology who are indicated for PKP or PPV, AGV implantation can be performed simultaneously.
Figures and Tables
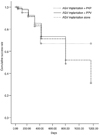 | Fig. 1The cumulative success rates of the three groups determined by Kaplan Meier analysis. AGV = Ahmed glaucoma valve; PKP = penetrating keratoplasty; PPV = pars plana vitrectomy. |
Table 1
Baseline characteristics of all patients

PKP = penetrating keratoplasty; AGV = Ahmed glaucoma valve; PPV = pars plana vitrectomy; IOP = intraocular pressure; SACG = secondary angle closure glaucoma; SOAG = secondary open angle glaucoma; ICE = iridocorneal endothelial; NVG = neovascular glaucoma; PACG = primary angle closure glaucoma; PXG = pseudoexfoliation glaucoma.
References
1. Kirkness CM, Steele AD, Ficker LA, Rice NS. Coexistent corneal disease and glaucoma managed by either drainage surgery and subsequent keratoplasty or combined drainage surgery and penetrating keratoplasty. Br J Ophthalmol. 1992. 76:146–152.
2. Arroyave CP, Scott IU, Fantes FE, et al. Corneal graft survival and intraocular pressure control after penetrating keratoplasty and glaucoma drainage device implantation. Ophthalmology. 2001. 108:1978–1985.
3. Al-Torbak A. Graft survival and glaucoma outcome after simultaneous penetrating keratoplasty and ahmed glaucoma valve implant. Cornea. 2003. 22:194–197.
4. Rapuano CJ, Schmidt CM, Cohen EJ, et al. Results of alloplastic tube shunt procedures before, during, or after penetrating keratoplasty. Cornea. 1995. 14:26–32.
5. Kwon YH, Taylor JM, Hong S, et al. Long-term results of eyes with penetrating keratoplasty and glaucoma drainage tube implant. Ophthalmology. 2001. 108:272–278.
6. Coleman AL, Mondino BJ, Wilson MR, Casey R. Clinical experience with the Ahmed glaucoma valve implant in eyes with prior or concurrent penetrating keratoplasties. Am J Ophthalmol. 1997. 123:54–61.
7. Faghihi H, Hajizadeh F, Mohammadi SF, et al. Pars plana Ahmed valve implant and vitrectomy in the management of neovascular glaucoma. Ophthalmic Surg Lasers Imaging. 2007. 38:292–300.
8. Lee KS, Sung KR, Na JH, et al. Clinical results of modified anterior chamber tube shunt to an encircling band surgery for uncontrolled intraocular pressure. J Glaucoma. 2011. 06. 22. [Epub]. DOI: 10.1097/IJG.0b013e318225b428.
9. Minckler DS, Heuer DK, Hasty B, et al. Clinical experience with the single-plate Molteno implant in complicated glaucomas. Ophthalmology. 1988. 95:1181–1188.
10. Coleman AL, Hill R, Wilson MR, et al. Initial clinical experience with the Ahmed glaucoma valve implant. Am J Ophthalmol. 1995. 120:23–31.
11. Lloyd MA, Baerveldt G, Fellenbaum PS, et al. Intermediate-term results of a randomized clinical trial of the 350- versus the 500-mm2 Baerveldt implant. Ophthalmology. 1994. 101:1456–1463.
12. Lavin MJ, Wormald RP, Migdal CS, Hitchings RA. The influence of prior therapy on the success of trabeculectomy. Arch Ophthalmol. 1990. 108:1543–1548.
13. Brandt JD, Wittpenn JR, Katz LJ, et al. Conjunctival impression cytology in patients with glaucoma using long-term topical medication. Am J Ophthalmol. 1991. 112:297–301.
14. Baudouin C, Garcher C, Haouat N, et al. Expression of inflammatory membrane markers by conjunctival cells in chronically treated patients with glaucoma. Ophthalmology. 1994. 101:454–460.
15. Broadway DC, Grierson I, O'Brien C, Hitchings RA. Adverse effects of topical antiglaucoma medication. II. The outcome of filtration surgery. Arch Ophthalmol. 1994. 112:1446–1454.
16. Broadway DC, Grierson I, O'Brien C, Hitchings RA. Adverse effects of topical antiglaucoma medication. I. The conjunctival cell profile. Arch Ophthalmol. 1994. 112:1437–1445.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download