Abstract
Purpose
The purpose of this study was to investigate the myotoxicity of bevacizumab on extraocular muscles in a rabbit model.
Methods
Thirty New Zealand white rabbits were used for this study. The animals were evenly divided into two groups. In the first group, 15 rabbits were treated with intramuscular injections of bevacizumab (1.25 mg/0.05 mL) in the right superior rectus muscle and normal saline solution (0.05 mL) in the left superior rectus muscle. In the second group, 15 rabbits were treated with subconjunctival injections of bevacizumab (2.5 mg/0.1 mL) in the right superior subconjunctival area and normal saline solution (0.1 mL) in the left superior subconjunctival area. Five rabbits in each group were sacrificed at one day, two weeks and four weeks after the injections. Extraocular muscle samples were prepared for light microscopic (LM) and electron microscopic (EM) examination. Degrees of acute inflammation were evaluated via CD-11b immunohistochemistry, and global muscle change was investigated using hematoxylin and eosin stains. Intensity of fibrosis was evaluated using Masson trichrome stains, and ultrastructural changes were observed on EM.
Bevacizumab (Avastin; Genentech Inc., San Francisco, CA, USA) is a complete, humanized monoclonal antibody directed against all isoforms of vascular endothelial growth factor (VEGF). Bevacizumab was originally developed as a treatment for metastatic colorectal cancer [1], and it has been successfully applied off-label for intravitreal treatment of VEGF-mediated ocular diseases such as choroidal neovascular disorder, central retinal vein occlusion, proliferative diabetic retinopathy, and pseudophakic cystoid macular edema [2]. Recently, bevacizumab has been introduced as a new therapeutic strategy for many other ocular disorders, such as recurrent pterygium, neovascularization caused by chemical burns, viral infections of the cornea and bleb survival after glaucoma surgery [3-7]. Therefore, bevacizumab can be used to treat numerous ocular disorders, and it has been applied by various administration routes: anterior chamber injection, topical application or subconjunctival bevacizumab injection [3-7].
However, there have been many concerns regarding potential ocular side effects of bevacizumab. Several drugs that have been applied in ophthalmic treatments have demonstrated extraocular muscle toxicity. Anesthetics such as levobupivacaine and bupivacaine induce morphopathological changes in extraocular muscle [8,9]. However, subconjunctival injection of commercially available gentamicin and amphotericin causes acute toxic reactions in the extraocular muscles [10,11].
Despite this finding, other reports have demonstrated the safety profile of bevacizumab on the retina and cornea [12-17]; no data are currently available regarding the safety profile of bevacizumab on extraocular muscles. Recently, paralytic strabismus has been reported after bevacizumab injection in ocular disorders [18,19]. Therefore, this study was designed to evaluate the histological muscle safety of bevacizumab when injected directly into the extraocular muscle and subsequently diffused subconjunctivally in a rabbit model.
Thirty adult New Zealand white rabbits (2.0 to 3.0 kg, 20 weeks old) were used for this study. All rabbits were confirmed to be free from ocular disease. The animals were handled in accordance with the Association for Research and Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research.
All surgical procedures were performed identically in both experimental and control eyes by a single surgeon (JHJ) as follows. In the first group (15 rabbits), the animals were treated with intramuscular injections of bevacizumab (1.25 mg/0.05 mL) in the right superior rectus muscle and with normal saline (0.05 mL) in the left superior rectus muscle. In the second group (15 rabbits), animals received subconjunctival injections of bevacizumab (2.5 mg/0.1 mL) in the superior conjunctiva of the right eye and normal saline (0.1 mL) subconjunctival injections in the left eye. Each rabbit was anesthetized with ketamine (400 mg/kg; Huons, Seoul, Korea) and xylazine (5 mg/kg; Bayer, Seoul, Korea) and placed in a stereotactic frame. The eyelids were opened using a speculum, and proparacaine was dropped into the conjunctival cul-de-sac. In the first group, the superior conjunctiva and Tenon's capsule were opened, and the superior rectus muscle was isolated from other tissues using cotton swabs and a muscle hook. Intramuscular injections were performed under direct visualization of the superior rectus muscle. After exposure, the superior rectus muscle was grasped with forceps, and a 30-gauge needle was placed 5 mm behind the insertion of the superior rectus muscle along the direction of the muscle fiber, and bevacizumab or normal saline was injected slowly. In the second group, subconjunctival injections were performed 3 mm behind the superior corneal limbus. The needle was removed 30 seconds after completion of injection to allow diffusion of the bevacizumab into the subconjunctival space and muscle and also to reduce leakage in both groups. After surgery, 3 mg/mL of ofloxacin (Tarivid; Santen, Osaka, Japan) eye drops were administered in each eye in both groups. All animals were observed and examined regularly for evidence of gross inflammatory reactions and scar formation by an investigator (HYC) in a blinded fashion. The animals were randomly sacrificed with an overdose of barbiturate anesthesia either one day, two weeks or four weeks post operation, and all eyes were enucleated and examined.
After sacrifice, the sites of muscle injection (5 mm behind the muscle insertion) and subconjunctival injection (3 mm behind the muscle insertion) were processed for light microscopic histopathologic evaluation of acute inflammatory reactions and late fibrotic scar formation of muscle tissue.
One day postoperatively, acute inflammatory reactions were evaluated in five rabbits from each group. Sections were stained with hematoxylin and eosin (H&E) for general histologic observation and also with antibodies for CD 11b that were specific for inflammatory cells: macrophages, monocytes and neutrophils. We performed H&E staining to assess global histologic changes of both groups two weeks after surgery.
Both eyes from every rabbit were evaluated for post inflammatory fibrotic scar formation and global histologic changes of each group four weeks postoperatively. Isolated tissues were examined using Masson's trichrome staining for scar tissue formation and H&E staining for general histologic observation. The fibrosis was graded based on the amount of collagen formation as follows: 0 = no fibrosis; 1 = mild perimuscular fibrotic reaction (stained collagen was detectable only in thin bands immediately adjacent to the muscle); 2 = easily detected thick bands; 3 = well-developed, dense bands of collagen; and 4 = a severe fibrotic response replacing large areas [20].
We investigated ultra-structural muscle tissue changes after bevacizumab muscle injection (one day and four weeks after surgery) by electron microscope. The specimens were pre-fixed with 2.5% glutaraldehyde (4℃, phosphate buffer, pH 7.2) and were post-fixed with 1% osmium tetroxide in the same buffer. The materials were dehydrated with a graded ethyl alcohol series and embedded in epoxy resin (Epon 812 mixture). Thick sections (1 µm) were stained with 1% toluidine blue for light microscope examination. Thin sections (50 to 60 nm) were prepared by an ultramicrotome (Reichert SuperNova; Leica, Wein, Austria) and were double stained with uranyl acetate and lead citrate. Thin sections were also examined with a transmission electron microscope (JEM 1200EX-II; JEOL, Tokyo, Japan).
The rabbits were monitored daily for changes within the orbit. All rabbits appeared to be healthy and ate normally. Mild conjunctival redness developed at the surgical site in some of the eyes in the first group. However, no significant ocular, periocular or orbital changes were noted in the treated eyes, and no gross changes were visible in the treated muscles at necropsy. There was no evidence of systemic toxicity in any animal.
In the first group (muscle injection group), the extraocular muscle layers in all eyes were intact with no evidence of tissue injury or muscle fiber disorganization. Only minor edema occurred in sites that received direct needle penetration. The muscle fibers lacked hyper-contracted muscle fiber, showed even staining and were of uniform size in diameter with arrangement. However, there was minimal influx of CD-11 positive cells into the connective tissue of the extraocular muscle after direct muscle injections of bevacizumab and normal saline one day after the procedure. These cellular responses were of the same degree without significant differences between eyes that received bevacizumab injection and normal saline injection (Fig. 1).
Two weeks after the injections, we did not observe tissue necrosis or inflammatory cell infiltration in eyes that received bevacizumab and normal saline muscle injections; there were no remarkable differences between experimental and control eyes.
Masson trichrome staining revealed that the median value of fibrosis grading in the bevacizumab injection group and the normal saline injection group was 0 (no fibrosis), and the capillary networks and muscle global appearance of each muscle section were intact four weeks after bevacizumab injection and normal saline injection (Fig. 2).
In the second group (subconjunctival injection group), we observed no inflammatory cellular infiltration in H&E staining and no significant influx of CD-11 positive cells into muscle or connective tissue in eyes that received bevacizumab and normal saline injections one day after the procedure. There was no evidence of muscle necrosis and fibrosis in any of the sections from eyes that received bevacizumab and normal saline subconjunctival injections at two and four weeks after the procedure (Fig. 3).
Electron microscopic findings revealed normal muscles in all eyes. The nuclei and cell membranes remained morphologically unaffected, and the distinct banding patterns of sarcomeres were intact after the injections. Myofibrils and tubular systems were maintained, and mitochondria seemed to be preserved. In addition, the appearance of myonuclei was within the normal range, and we observed no significant differences between the control groups. The vascular beds, nerve endings and satellite cells appeared intact, and no significant differences were noted at all times in all sections (Fig. 4).
The histological characteristics of mammalian extraocular muscles, which perform the functions of both red and white muscles, differ in many respects from those of other striated muscles. Extraocular muscle is distinguished by the finest fibers of any striated muscles, and the nerve supply to the extraocular muscles is astonishingly rich [21]. Extraocular muscles vary in diameter from 9 to 17 µm [22] and contain different types of muscle fibrils with intricate, ultramicroscopic structures and fibers with highly differentiated nerve endings [22,23]. Physiologically, extraocular muscle requires and receives more oxygen than other skeletal muscles and contracts much more quickly than other voluntary muscles [24]. All of these characteristics of extraocular muscles are related to fine extraocular muscle regulation, and any muscle injuries caused by extrinsic agents can lead to extraocular muscle dysfunction.
Some drugs that have been used in the ophthalmic arena have produced extraocular muscle toxicity [8-11]. Although the molecular patho-mechanisms of each drug are different, they follow similar histopathological courses. In acute stages, inflammatory cell infiltration is observed in muscle tissue, and necrobiotic changes may follow, ranging from fiber vacuolation and myocyte edema up to total disintegration of intracellular structures and myonecrosis [25,26]. Subsequently, satellite cells become activated, and muscle fibers are regenerated; in the final stages of inflammation, injured muscle demonstrates muscle atrophy and surrounding tissue fibrosis [27,28].
The present study found no significant tissue destruction on H&E staining or tissue fibrosis on Masson's trichrome stain after intramuscular and subconjunctival bevacizumab injection. CD-11b immunohistochemistry revealed no significant inflammatory cell infiltration in extraocular muscle tissue compared to control eyes. Although the intramuscular bevacizumab and normal saline injection groups showed minimal inflammatory cell infiltration in the connective tissue during the acute phase, this infiltration spontaneously disappeared and was probably related to direct needle injury or to the surgical procedure itself. Electron microscopic findings also revealed no significant necrosis of the muscle fiber and no remarkable vascular changes.
There are many ocular disorders in which VEGF plays a major role, such as retinal and choroidal vasoproliferative disorders, neovascular glaucoma and corneal neovascularization. Furthermore, VEGF is a vital factor in the inflammatory process and wound healing response [29,30], and it is present in extraocular muscle and retroorbital tissue [31]. Therefore, we expect that bevacizumab will be a useful future treatment modality in intraocular or extraocular ocular disorders.
In the current study, we observed no evidence of muscle injury or vascular network damage in any of the sections examined. Nevertheless, a normal histological appearance does not rule out functional changes to extraocular muscles. The effects of repeated exposure and the half-life of bevacizumab in the extraocular muscle are unknown; therefore, further studies will be required to evaluate the long-term safety of this drug and the possibility of functional side effects. In summary, we believe that the conventional dose of bevacizumab induced no extraocular muscle toxicity and was a safe agent for extraocular muscle.
Figures and Tables
 | Fig. 1One day following muscle injection. (A) Microscopic findings revealed no remarkable inflammatory cell infiltration in a rabbit after receiving bevacizumab injection. (B) The same degree of inflammatory cells is present in the control eye (H&E, ×200). (C) CD-11b antibody immunostaining of the extraocular muscle. Note the lack of remarkable staining of CD-11b-positive inflammatory cells (which stain a brown or black color, and indicates the presence of monocytes, macrophages and neutophils) infiltration in the muscle layer of a bevacizumab injection in the rabbit. (D) Control group showing the no significant degree of CD-11b-positive cellular response (×200). |
 | Fig. 2Four weeks after muscle injection of bevacizumab (A) and normal saline (B). The muscle shows a uniform diameter, even staining and unremarkable fibrosis compared to the control eye (Masson's trichrome, ×100). |
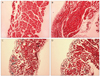 | Fig. 3Subconjunctival injection group. (A) One day following bevacizumab injection in the subconjunctival space. Microscopic findings reveal regularly arranged individual muscle fibers surrounded by thin fibrous connective tissues and blood vessels (H&E, ×200). (B) Normal appearance in a normal saline injection eye (H&E, ×200) one day after injection. (C,D) Four weeks after bevacizumab and normal saline injections, a small amount of collagen fibers and minimal fibrosis are present in surrounding tissue (Masson's trichrome, ×100). |
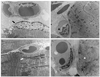 | Fig. 4Electron micrograph of extraocular muscle after bevacizumab muscle injection. (A) Longitudinal section of myofiber one day following bevacizumab muscle injection. Normal, ultra structural appearance of muscle fiber with subsarcolemmal nuclei is observed (black arrow). The distinct and intact banding patterns of sarcomeres and the Z-band are visible (×3,000). (B) Transverse section of the muscle and capillaries one day after bevacizumab muscle injection. Endothelial cells of the capillaries exhibit normal cytology, and the capillaries show intact structural integrity (black arrow head) (×3,000). (C) Longitudinal section of a myofiber four weeks after bevacizumab muscle injection. Muscle fibers and mitochondrial membranes (white arrow) are seen (×3,000). (D) Four weeks after bevacizumab injection, capillary endothelium and connective tissue of the muscle layer are intact. Dense and regular, uniform arrayed muscle fibers are seen (white arrow head) (×3,000). |
Acknowledgements
We would like to thank Sang-Sik Kim for his technical support in the electron-microscopy examination.
References
1. Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004. 350:2335–2342.
2. Gunther JB, Altaweel MM. Bevacizumab (Avastin) for the treatment of ocular disease. Surv Ophthalmol. 2009. 54:372–400.
3. Banifatemi M, Razeghinejad MR, Hosseini H, Gholampour A. Bevacizumab and ocular wound healing after primary pterygium excision. J Ocul Pharmacol Ther. 2011. 27:17–21.
4. Oh JY, Kim MK, Wee WR. Subconjunctival and intracorneal bevacizumab injection for corneal neovascularization in lipid keratopathy. Cornea. 2009. 28:1070–1073.
5. Saravia M, Zapata G, Ferraiolo P, et al. Anti-VEGF monoclonal antibody-induced regression of corneal neovascularization and inflammation in a rabbit model of herpetic stromal keratitis. Graefes Arch Clin Exp Ophthalmol. 2009. 247:1409–1416.
6. Dastjerdi MH, Al-Arfaj KM, Nallasamy N, et al. Topical bevacizumab in the treatment of corneal neovascularization: results of a prospective, open-label, noncomparative study. Arch Ophthalmol. 2009. 127:381–389.
7. Memarzadeh F, Varma R, Lin LT, et al. Postoperative use of bevacizumab as an antifibrotic agent in glaucoma filtration surgery in the rabbit. Invest Ophthalmol Vis Sci. 2009. 50:3233–3237.
8. Okland S, Komorowski TE, Carlson BM. Ultrastructure of mepivacaine-induced damage and regeneration in rat extraocular muscle. Invest Ophthalmol Vis Sci. 1989. 30:1643–1651.
9. Kytta J, Heinonen E, Rosenberg PH, et al. Effects of repeated bupivacaine administration on sciatic nerve and surrounding muscle tissue in rats. Acta Anaesthesiol Scand. 1986. 30:625–629.
10. Chapman JM, Abdelatif OM, Cheeks L, Green K. Subconjunctival gentamicin induction of extraocular toxic muscle myopathy. Ophthalmic Res. 1992. 24:189–196.
11. O'Day DM, Smith R, Stevens JB, et al. Toxicity and pharmacokinetics of subconjunctival amphotericin B: an experimental study. Cornea. 1991. 10:411–417.
12. Lim TH, Bae SH, Cho YJ, et al. Concentration of vascular endothelial growth factor after intracameral bevacizumab injection in eyes with neovascular glaucoma. Korean J Ophthalmol. 2009. 23:188–192.
13. Kernt M, Welge-Lussen U, Yu A, et al. Bevacizumab is not toxic to human anterior- and posterior-segment cultured cells. Ophthalmologe. 2007. 104:965–971.
14. Yoeruek E, Spitzer MS, Tatar O, et al. Safety profile of bevacizumab on cultured human corneal cells. Cornea. 2007. 26:977–982.
15. Bakri SJ, Cameron JD, McCannel CA, et al. Absence of histologic retinal toxicity of intravitreal bevacizumab in a rabbit model. Am J Ophthalmol. 2006. 142:162–164.
16. Manzano RP, Peyman GA, Khan P, Kivilcim M. Testing intravitreal toxicity of bevacizumab (Avastin). Retina. 2006. 26:257–261.
17. Kaempf S, Johnen S, Salz AK, et al. Effects of bevacizumab (Avastin) on retinal cells in organotypic culture. Invest Ophthalmol Vis Sci. 2008. 49:3164–3171.
18. Cakmak HB, Toklu Y, Yorgun MA, Simsek S. Isolated sixth nerve palsy after intravitreal bevacizumab injection. Strabismus. 2010. 18:18–20.
19. Park HJ, Guy J. Sixth nerve palsy post intravitreal bevacizumab for AMD: a new possibly causal relationship and complication? Binocul Vis Strabismus Q. 2007. 22:209.
20. Mora JS, Sprunger DT, Helveston EM, Evan AP. Intraoperative sponge 5-fluorouracil to reduce postoperative scarring in strabismus surgery. J AAPOS. 1997. 1:92–97.
21. Ruff R, Kaminski H, Maas E, Spiegel P. Ocular muscles: physiology and structure-function correlations. Bull Soc Belge Ophtalmol. 1989. 237:321–352.
22. Chiarandini DJ, Davidowitz J. Structure and function of extraocular muscle fibers. Curr Top Eye Res. 1979. 1:91–142.
23. Merrillees NC, Sunderland S, Hayhow W. Neuromuscular spindles in the extraocular muscles in man. Anat Rec. 1950. 108:23–30.
24. Tengroth B, Hamberger A. Oxygen consumption studies on guinea pigs after administration of D- and L-Thyroxine: effect on the whole animal compared with that on its eye muscles. Acta Ophthalmol (Copenh). 1963. 41:524–530.
25. Komorowski TE, Shepard B, Okland S, Carlson BM. An electron microscopic study of local anesthetic-induced skeletal muscle fiber degeneration and regeneration in the monkey. J Orthop Res. 1990. 8:495–503.
26. Zink W, Graf BM. Local anesthetic myotoxicity. Reg Anesth Pain Med. 2004. 29:333–340.
27. Tidball JG. Inflammatory cell response to acute muscle injury. Med Sci Sports Exerc. 1995. 27:1022–1032.
28. Cotran RS, Kumar V, Robbins SL. Robbins SL, Cotran RS, Kumar V, editors. Inflammation and repair. Robbins' pathologic basis of disease. 1989. 4th ed. Philadelphia: Saunders;39–71.
29. Hoeben A, Landuyt B, Highley MS, et al. Vascular endothelial growth factor and angiogenesis. Pharmacol Rev. 2004. 56:549–580.
30. Wilgus TA, Ferreira AM, Oberyszyn TM, et al. Regulation of scar formation by vascular endothelial growth factor. Lab Invest. 2008. 88:579–590.
31. Matos K, Manso PG, Marback E, et al. Protein expression of VEGF, IGF-1 and FGF in retroocular connective tissues and clinical correlation in Graves' ophthalmopathy. Arq Bras Oftalmol. 2008. 71:486–492.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download