Abstract
Purpose
To compare the histopathologic and morphologic findings of encapsulated blebs following Ahmed glaucoma valve implantation and primary standard trabeculectomy with mitomycin-C.
Methods
We reviewed the records of patients with otherwise uncontrollable glaucoma who had undergone Ahmed glaucoma valve implantation or trabeculectomy with mitomycin-C. Five eyes that underwent Ahmed valve implantation and three eyes that underwent trabeculectomy needed surgical revision of the initial surgery due to encapsulated bleb development with total loss of function. The surgically removed encapsulated blebs were analyzed macroscopically and microscopically.
Results
Removal of the encapsulated bleb was performed at a mean follow-up time of 26.6 ± 19.4 weeks in the Ahmed valve implantation group and 12.0 ± 11.4 weeks in the trabeculectomy group. The fibrotic wall of the encapsulated blebs had an overall thickness of 2.48 ± 0.42 mm in the Ahmed valve implantation group and 1.62 ± 0.37 mm in the trabeculectomy group. Macroscopically, the coconut flesh-like smooth surface was split into two layers, and the wall of the capsule was thicker in the Ahmed valve implantation group than in the trabeculectomy group. Histopathologically, the fibrotic capsule was composed of an inner fibrodegenerative layer and an outer fibrovascular layer, and there were no histopathological differences between the two groups.
In patients with medically uncontrollable glaucoma, surgical management is necessary. The two surgical treatments with the highest rates of success are trabeculectomy with adjunctive antimetabolic therapy and implantation of glaucoma drainage devices such as an Ahmed valve. Despite the enormous improvements in surgical techniques, there are a number of possible complications following surgical procedures. Encapsulated bleb (EB) formation, which is a common cause of drainage failure in the early post-operative period, is still a prominent issue for ophthalmic surgeons. This finding is usually accompanied by elevated intraocular pressure. Therefore, the clinical success of glaucoma drainage devices is often impaired due to the development of EB around the antiglaucoma device or scleral flap, thus requiring further interventions. In previous studies, the incidence of bleb encapsulation has ranged from 2.5% to 29% with various surgical techniques in different types of glaucoma [1-4].
This study was designed to compare the histopathologic and morphologic findings of EB following two distinctive surgeries, Ahmed glaucoma valve implantation and trabeculectomy with mitomycin-C.
Eight consecutive patients who underwent EB removal at Samsung Medical Center between February 2009 and July 2010 were studied retrospectively. The patients with uncontrolled and complicated glaucoma had already undergone conventional surgical treatment (Trabeculectomy, Ahmed valve implantation). The trabeculectomies were performed using Watson's modification of the Cairns' technique described in detail elsewhere [5,6]. A fornix-based conjunctival flap was created, a square 4 × 4 mm scleral flap was prepared, and a 1 × 2 mm trabeculectomy was followed by a peripheral iridectomy. The scleral flap was closed with two single 10-0 nylon monofilament sutures at the corners, while Tenon's capsule and the conjunctival wound were closed separately with running 10-0 nylon sutures. Subconjunctival injections of gentamicin and dexamethasone were delivered 180° from the operation site. Soaking of a Wexel sponge dipped in mitomycin-C 0.04% over the sclera flap was performed for 150 seconds. Meanwhile, an Ahmed valve implant with a polypropylene base plate was inserted using a standard technique. A fornix-based conjunctival flap was created in the superotemporal or superonasal quadrant, and the adjacent recti muscles were identified. The polypropylene body was placed 8 to 10 mm posterior to the corneoscleral limbus and sutured to the globe with 9-0 nylon sutures. The tube was trimmed to the appropriate length and inserted into the anterior chamber at the limbus through a paracentesis site created with a 23-gauge needle. Donor human sclera was trimmed, placed over the tube, and secured to the globe with nylon sutures. The conjunctiva was closed with an 8-0 Vicryl suture. Subconjunctival injections of gentamicin and dexamethasone were delivered 180° from the operation site. No antimetabolite was used during the surgical procedure. Topical antibiotics were prescribed four times a day, and topical steroids were administered for two months postoperatively through a dose-tapering regimen.
During the follow-up period, the uncontrolled glaucoma patients were treated with antiglaucoma medications and digital ocular compression. Transconjunctival needling with 5-fluorouracil or mitomycin-C injections was performed when medical therapy did not reduce the IOP to a level adequate for the individual patient. An EB was defined as an elevated, smooth-surfaced, tense, thickened, dome-shaped fibrotic membrane [1]. EB removal was carried out under local/general anesthesia by an experienced surgeon (CK) when the above-mentioned treatments failed to control the intraocular pressure (IOP) level. The following information was documented for each patient: age, gender, ophthalmologic history, type of glaucoma, time from glaucoma surgery to EB removal, pre-operative treatment, prescribed anti-glaucoma medications, conjunctival flap, and baseline, pre-operative, and post-operative intraocular pressures.
During surgery, the conjunctiva and tenon were dissected, and the EB was then directly opened. The fibrotic capsule was carefully removed along with as much tissue as possible. The wound was then closed using 8-0 vicryl sutures. Immediately after resection, the specimens were fixed in formalin and transferred to the laboratory for further processing. A gross picture of the specimen was taken, and the specimen was then embedded in paraffin and epoxy resin according to standard protocols. The paraffin sections were stained with hematoxylin-eosin, trichrome Masson-Goldner, and alcian blue stains to observe the collagen fibers and to count the number of fibroblasts in the hyperfield area.
In total, we studied three eyes from three patients who underwent trabeculectomy with mitomycin-C and five eyes from four patients who underwent Ahmed valve implantation before the identification of bleb encapsulation. The underlying diagnoses were congenital glaucoma (2 eyes), aphakic glaucoma after retina surgery (1 eye), secondary glaucoma associated with pseudophakia (1 eye), secondary glaucoma associated with Cogan-Reese syndrome (1 eye), secondary glaucoma after penetrating keratoplasty (2 eyes), and neovascular glaucoma (1 eye). The clinical data related to these patients is included in Table 1. On average, EB removal was performed at 26.6 ± 19.4 weeks after the Ahmed valve implantation procedure and 12.0 ± 11.4 weeks after trabeculectomy with mitomycin C. The mean intraocular pressure of 31.20 ± 5.26 mmHg at diagnosis decreased to 11.80 ± 5.12 mmHg after the removal of the EB in the Ahmed valve implantation group, and 30.67 ± 11.02 mmHg at diagnosis decreased to 9.33 ± 3.06 mmHg post-operatively in the trabeculectomy group.
The macroscopic analysis of the excised capsule revealed a white, smooth, coconut flesh-like inner surface and an irregular vascularized outer surface in both groups (Fig. 1). The capsular wall was thicker in the Ahmed valve implantation group (2.48 ± 0.42 mm) compared to the trabeculectomy group (1.62 ± 0.37 mm). Histopathologically, the subdivision of the tissue was also evident; the vascular structure of the outer layer resembled normal collagenous connective tissue containing small amounts of elastin, and the inner surface consisted of dense collagen fibers with elastoid degeneration (Figs. 2, 3, 4). An inflammatory response was nearly absent in all areas of the excised specimen. The ratio of outer fibrovascular layer thickness to inner fibrodegenerative layer thickness was approximately 3 : 1, and there was no difference between the two groups (Fig. 5).
In a number of surgical cases, EB develop around the antiglaucoma device or scleral flap after glaucoma surgery, requiring further surgical intervention. The process of EB formation is not well understood, but the migration and proliferation of fibroblasts and aqueous humor seem to play a major role in EB formation. Fibroblasts activated by the inflammatory reaction as part of the healing process trigger scar formation, and the occlusion of aqueous humor outflow as a result of excessive inflammation is a major cause of early failure of glaucoma surgery [7]. Also, aqueous humor displacement of interstitial tissue fluid from the inner surface of the bleb capsule may induce gradual cell death followed by collagen fiber swelling, fragmentation, and opening of the intercellular matrix.
Scarring of Tenon's capsule localized at the filtration site with a specific dome-shaped, hard appearance associated with IOP elevation were initially reported by Van Buskirk in eight patients who had undergone a variety of filtering operations [1]. In previous studies, the incidence of bleb encapsulation ranged from 2.5% to 29% with various surgical techniques in different types of glaucoma. Comparing these studies, it is important to consider the possible effect of surgical technique on the incidence rate. Factors such as trabeculectomy versus glaucoma device implantation and the use of an antimetabolic agent may influence this rate.
The surgical mechanisms for the two procedures compared in this study are quite different. In trabeculectomy, an aqueous outflow tract is created on the patient's sclera, and an antimetabolic agent is soaked over the area for a few minutes to prevent adhesion between the scleral flap and conjunctival reservoir. On the other hand, a polypropylene base plate and a sheet-like valve without an adjunctive antimetabolic agent are used in the Ahmed glaucoma valve implantation procedure. During insertion, the Ahmed glaucoma valve is fixed to the sclera and covered by the tenon and conjunctiva, forming a filtering bleb far from the limbal region.
The indicated types of glaucoma are also different. Trabeculectomy is the treatment of choice in open angle glaucoma, but the majority of studies involving glaucoma drainage devices include patients with neovascular or uveitic glaucoma.
Thus far, there are only a few published studies on encapsulation in trabeculectomy, and there has not been a comparative study on encapsulation following Ahmed glaucoma valve implantation or trabeculectomy with mitomycin-C [3,4,8,9]. In our study, macroscopic evaluation revealed that the EB was significantly thicker in the Ahmed valve implantation group compared to the trabeculectomy group. On the other hand, the histologic findings of the EB were similar to findings from previous studies [7,10,11]. The EB was subdivided into two layers, an outer fibrovascular layer and an inner fibrodegenerative layer with dense collagen fibers. There were no differences in the histologic findings between the two groups, suggesting a common pathophysiologic pathway of EB formation, as is also observed in other healing tissues.
The use of adjunctive mitomycin-C could be the causative factor explaining the difference in capsule wall thickness. mitomycin-C was reported to lower the rate (2.5%) of encapsulation in one series of trabeculectomies [3]. However, Campagna et al. [4] reported a 29% incidence with mitomycin-C in another series of 100 patients. Nevertheless, mitomycin-C may reduce the activity of fibroblasts in the trabeculectomy group and may lead to reductions in EB wall thickness.
Previous data show that the base plate material used in Ahmed implants can play an important role in fibrous tissue formation and may be involved in encapsulation. Polypropylene, which was used in early Ahmed glaucoma base plates, seems to have a higher potency for encapsulation than the later silicone base plates [12]. A polypropylene base plate was used in all patients who underwent Ahmed valve implantation in the present study. The new material was not sufficient for preventing formation of encapsulation, indicating that the base plate material may not be the most important factor in triggering fibrous tissue development.
Further investigations, such as electron microscopy or other special staining techniques, are needed to gain more information about EB. Electron micrographs of connective tissue may give us more precise information of epithelial cells, fibroblasts, and small blood vessels. Activated myofibroblasts and myofibrils within the inner layer, compared with the narrow silent fibrocytes in the outer layer, were reported from electron microscopy following trabeculectomy [11]. Collagen-specific staining like aldehyde fuchsin may show a loss of collagen in the inner layer of the EB, and immunostaining may indicate the activation and transformation of fibroblasts into myofibroblasts in the inner section [10].
Figures and Tables
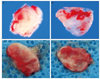 | Fig. 1Gross photographs of excised EB following trabeculectomy (A, outer surface; B, inner surface), and Ahmed valve implantation (C, outer surface; D, inner surface). |
 | Fig. 2Magnified photomicrograph of the encapsulated bleb following Ahmed valve implantation stained with H&E (×400). The vascular structure of the outer layer resembled normal collagenous connective tissue, and the inner surface consisted of dense collagen fibers with elastoid degeneration (arrow). |
 | Fig. 3Magnified photomicrograph of the encapsulated bleb following Ahmed valve implantation stained with Masson's trichrome (×400). Note collagen fiber staining in the outer fibrovascular portion of the bleb and fibrobastic proliferation. |
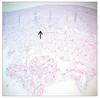 | Fig. 4Photomicrograph of the encapsulated bleb following trabeculectomy stained with Alcian blue (×100). Increased ground substance staining is present, especially around the cells (arrow). |
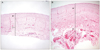 | Fig. 5Photomicrographs of the encapsulated bleb following trabeculectomy (A), and Ahmed valve implantation (B) showing fibrodegenerative changes in the inner layer (black arrowhead) and outer fibrovascular layer (white arrowhead) (H&E, ×100). The ratio of outer fibrovascular layer thickness to inner fibrodegenerative layer thickness was approximately 3 : 1 in both groups. |
Table 1
Details of demographics, ophthalmologic history, and ocular characteristics in eyes with encapsulated blebs

TRAB = trabeculectomy; OS = left eye; PE & PC-IOL = phacoemulsification & posterior chamber intraocular lens implantation; PKP = penetrating keratoplasty; MMC = mitomycin-C; α = α-agonist; β = β-blocker; CAI = carbonic anhydrase inhibitor; p = prostaglandin analog; OD = right eye; 5-FU = 5-fluorouracil; PPV = pars plana vitrectomy.
*Secondary glaucoma associated with pseudophakia; †Secondary glaucoma associated with Cogan Reese; ‡Secondary aphakic glaucoma after retina surgery; §Secondary glaucoma after penetrating keratoplasty; ∥Neovascular glaucoma; ¶Congenital glaucoma.
References
1. Van Buskirk EM. Cysts of Tenon's capsule following filtration surgery. Am J Ophthalmol. 1982. 94:522–527.
2. Richter CU, Shingleton BJ, Bellows AR, et al. The development of encapsulated filtering blebs. Ophthalmology. 1988. 95:1163–1168.
3. Azuara-Blanco A, Bond JB, Wilson RP, et al. Encapsulated filtering blebs after trabeculectomy with mitomycin-C. Ophthalmic Surg Lasers. 1997. 28:805–809.
4. Campagna JA, Munden PM, Alward WL. Tenon's cyst formation after trabeculectomy with mitomycin C. Ophthalmic Surg. 1995. 26:57–60.
5. Cairns JE. Trabeculectomy. Preliminary report of a new method. Am J Ophthalmol. 1968. 66:673–679.
6. Watson PG, Barnett F. Effectiveness of trabeculectomy in glaucoma. Am J Ophthalmol. 1975. 79:831–845.
7. Addicks EM, Quigley HA, Green WR, Robin AL. Histologic characteristics of filtering blebs in glaucomatous eyes. Arch Ophthalmol. 1983. 101:795–798.
8. Feldman RM, Gross RL, Spaeth GL, et al. Risk factors for the development of Tenon’s capsule cysts after trabeculectomy. Ophthalmology. 1989. 96:336–341.
9. Schwartz AL, Van Veldhuisen PC, Gaasterland DE, et al. The Advanced Glaucoma Intervention Study (AGIS). 5. Encapsulated bleb after initial trabeculectomy. Am J Ophthalmol. 1999. 127:8–19.
10. Molteno AC, Fucik M, Dempster AG, Bevin TH. Otago Glaucoma Surgery Outcome Study: factors controlling capsule fibrosis around Molteno implants with histopathological correlation. Ophthalmology. 2003. 110:2198–2206.
11. Thieme H, Choritz L, Hofmann-Rummelt C, et al. Histopathologic findings in early encapsulated blebs of young patients treated with the ahmed glaucoma valve. J Glaucoma. 2011. 20:246–251.
12. Eibschitz-Tsimhoni M, Schertzer RM, Musch DC, Moroi SE. Incidence and management of encapsulated cysts following Ahmed glaucoma valve insertion. J Glaucoma. 2005. 14:276–279.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download