Abstract
Purpose
To evaluate the expression of the Na+-K+-2Cl--cotransporter 2 (NKCC2) in the ischemic rat retina.
Methods
Retinal ischemia was induced by pressures 90 to 120 mmHg, above systemic systolic pressure. Immunohistochemistry and western blot analysis were performed.
Results
NKCC2 is expressed in the normal retina and its expression is increased by ischemia caused by intraocular pressure elevation. NKCC2 immunoreactivity was observed mainly in axon bundles of ganglion cells and horizontal cell processes in the retina. NKCC2 expression continuously increased with a peak value 3 days (to 415% of normal levels) after ischemic injury, and then gradually decreased to 314% of controls until 2 weeks post injury. The mean density of NKCC2-labeled ganglion cells per mm2 changed from 1,255 ± 109 in normal retinas to 391 ± 49 and 185 ± 37 at 3 days and 2 weeks after ischemia, respectively (p < 0.05), implying cell death of ganglion cells labeled with NKCC2.
The Na+-K+-2Cl--cotransporter (NKCC) belongs to the cation-chloride co-transporter family, which transports Na+, K+, and Cl- ions across the plasma membrane of cells with a stoichiometry of 1Na+ : 1K+ : 2Cl-. The NKCC plays an important role in salt secretion and absorption, cell volume regulation, and maintenance of intracellular Cl- concentrations [1-3]. Recently, the NKCC has been reported to be associated with the cell cycle, cellular proliferation, and apoptotic cell death in ischemic tissues [4-7].
Two isoforms of the NKCC have been identified. The NKCC1 isoform was initially discovered on the basolateral membrane of the shark rectal gland and is expressed in a wide variety of tissues, including epithelial cells and non-epithelial cells. The NKCC2 isoform is expressed mainly in the apical membrane of epithelial cells of the thick ascending limb of Henle's loop in the kidney [1-3].
It has been reported that the NKCC is related with cell damage in many ischemic models [8-11]. In cerebral ischemia, NKCC1 contributes to astrocyte swelling, causing excitatory amino acid release, resulting in neuronal cell death [8,9]. Thus, pharmacological inhibition of NKCC1 is neuroprotective in ischemic or traumatic injuries of the brain. During heart ischemia, sodium accumulates outside cells due to impairment of ion channels or transporters like Na+-K+-ATPase, Na+-H+ exchanger, and Na+-Ca2+ exchanger and enters into cells through the NKCC [10]. Inhibition of the NKCC can attenuate the ischemic rise in intracellular sodium and maintain higher levels of high energy phosphates, significantly improving functional recovery.
Previous studies of the retina demonstrated that the NKCC is not expressed in retinal ganglion cells except during developing development [12,13]. However, the presence of the NKCC2 and its role in the retina have not yet been described. To address these issues, we analyzed the expression of the NKCC2 in the retina and observed expression change after retinal ischemia induced by intraocular pressure elevation.
Thirty-five adult male albino Sprague-Dawley rats weighing 200 to 250 g were used for this study. Animals were anesthetized with 4% chloral hydrate (1 mL/100 g body weight). The pupils were dilated with 1% tropicamide eye drops. The intraocular pressure (IOP) was raised to 90 to 120 mmHg by cannulation of the anterior chamber with a 30-gauge needle connected to a hydrostatic pressure device, and the elevated IOP was maintained for 60 minutes. The applied pressure of 90 to 120 mmHg is in the range, or slightly above, systemic systolic pressure in the rat. Following removal of the cannula, recirculation began immediately and the IOP decreased to normal values within 5 minutes. Animals were sacrificed by an overdose of chloral hydrate at 12 hours, 24 hours, 3 days, 7 days, and 14 days after reperfusion. The anterior segments of the eyeballs were removed. For western blot analysis, retinal tissues were quickly dissected on an ice-cold plate, frozen on dry ice, and stored at -70℃. For immunocytochemistry and quantitative analysis, the eyecups were fixed by immersion in fixative (4% paraformaldehyde/0.2% picric acid in 0.1 M phosphate buffer [PB, pH 7.4]), for 2 to 3 hours. Following fixation, the retina was carefully dissected and transferred to 30% sucrose in PB, for 24 hours at 4℃. They were then frozen in liquid nitrogen, thawed, and rinsed in 0.01 M phosphate buffered saline (PBS, pH 7.4). The central region near the optic disc was excised, dehydrated in a series of graded alcohol, and embedded in wax.
Western blot analysis was performed on the retinal extracts, which were homogenized in 10 volumes of 20 mM Tris-HCl (pH 7.4), 150 mM NaCl, 1 mM EDTA, 1% Triton ×-100, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate (SDS), 0.02% sodium azide, 1 mM PMSF, and 5 µg/mL leupeptin. Protein concentration for each sample was assayed by the Lowry method, in duplicate, and the results were averaged. Duplicate sets of protein standards containing 0, 1, 3, 5, 10, 20, 40, and 60 mg of bovine serum albumin were assayed by the same method. The results were averaged and graphed to produce a linear equation that was used to estimate the protein contents in the retinal extracts. The optical density of each sample was measured at 660 nm, using a spectrometer (Spectronic 20; Bausch & Lomb, Rochester, NY, USA). Tissue samples corresponding to 25 g of total protein were heated at 100℃ for 10 minutes with an equivalent volume of sample buffer (containing 4% SDS and 10% mercaptoethanol) and loaded onto 10% polyacrylamide gels. The proteins were electrotransferred to a nitrocellulose membrane in Tris-glycine-methanol buffer. The membrane was blocked for 1 hour at room temperature in a blocking solution containing 5% nonfat dry milk, 0.05% Tween-20, and PBS. The membrane was then incubated for 15 hour at 4℃ with a goat polyclonal antibody directed against NKCC2 (1 : 1,000; Chemicon, Temencula, CA, USA) in the blocking solution. The membrane was rinsed with 0.05% Tween-20 in PBS for three washes of 10 minutes and incubated for 1 hour at room temperature in a 1 : 200 dilution of biotinylated donkey anti-rabbit IgG (Vector Laboratories, Burlingame, CA, USA). The blot was washed three times for 10 minutes each and then processed for analysis using an enhanced chemiluminescence detection kit (Amersham, Arlington Heights, IL, USA).
For the immunocytochemistry of the NKCC2, 50-µm-thick vibratome sections, prepared as described above, were used. Endogenous peroxidase activity was blocked by placing the sections in 1.4% H2O2 in methanol for 10 minutes. The sections were then incubated in 10% normal serum (Vector Laboratories) in PBS for 1 hour at room temperature in order to block nonspecific binding sites. Sections were then incubated in a solution of antibodies to the NKCC2 (1 : 1,000) for 1 day at 4℃. After washing in PBS for 45 minutes (3 × 15 minutes), the retinal tissues were incubated for 12 hours in biotinylated donkey anti-rabbit IgG (1 : 50, Vector Laboratories) with 0.5% Triton X-100 at 4℃, rinsed in PBS, and subsequently incubated in Avidin-biotin-peroxidase complex (Vector Laboratories) in PBS for 1 day at 4℃. Retinas were rinsed in two changes of PBS and three changes of 0.05M Tris-HCl buffer (TB, pH 7.4) for 5 minutes at room temperature, and were then incubated in 0.05% 3,3-diaminobenzidine tetrahydrochloride in TB for 10 minutes. Hydrogen peroxide was added to the incubation medium to a final concentration of 0.01% H2O2, and the container was gently shaken as the reaction proceeded. After 1.2 minutes of reaction, as determined by the degree of staining, the reaction was stopped with several washes of TB and PB. The stained tissues were mounted onto gelatin-coated slides and coverslipped with glycerol.
To identify whether cells demonstrating NKCC2 immunoreactivity were ganglion cells, double labeling techniques using antibodies against the NKCC2 and Thy1.1, a specific marker for ganglion cells, were performed. Sections were incubated overnight in monoclonal Thy1.1 antibody (1 : 500; Chemicon, Temencula, CA, USA) with 0.5% Triton X-100 in 0.1M PB at 4℃. Sections were rinsed for 30 minutes with 0.01 M PBS, and the retinal tissues were incubated for 2 hours in biotinylated donkey anti-mouse IgG (1 : 50, Vector Laboratories), rinsed in PBS, and subsequently incubated for 2 hours in biotinylated donkey anti-mouse FITC (1 : 50, Vector Laboratories). After rinsing for 30 minutes with 0.01 M PB and for 30 minutes with 0.01 M PBS and with subsequent incubation for 1 hour in normal donkey serum, the retina was incubated overnight in anti-NKCC2 at 4℃. Sections were then rinsed for 30 minutes with 0.01 M PBS and incubated for 2 hour in biotinylated donkey anti-rabbit IgG (1 : 50, Vector Laboratories), rinsed in PBS, and incubated for 2 hour in biotinylated Cy-3 (1 : 500, Vector Laboratories).
To identify whether cells demonstrating NKCC2 immunoreactivity were horizontal cells, double labeling techniques using antibodies against the NKCC2 and calbindin, a specific marker for horizontal cells, were performed. After incubation overnight in monoclonal calbindin antibody (1 : 3,000, Chemicon) with 0.5% Triton X-100 in 0.1 M PB at 4℃, sections were incubated for 2 hours in biotinylated donkey anti-mouse FITC (1 : 50, Vector Laboratories), rinsed for 30 minutes with 0.01 M PB and 30 minutes with 0.01 M PBS, and then incubated for 2 hours in biotinylated donkey anti-mouse IgG (1 : 50, Vector Laboratories) again. We incubated retinal tissues overnight in biotinylated donkey anti-mouse FITC (1 : 50, Vector Laboratories) and rinsed for 30 minutes with 0.01 M PB and 30 minutes with 0.01 M PBS. In order to inhibit nonspecific reactions, sections were incubated in normal donkey serum for 1 hour. After overnight incubation in anti-NKCC2 at 4℃, sections were rinsed for 30 minutes with 0.01 M PBS. The retinal tissues were incubated for 2 hours in biotinylated donkey anti-rabbit IgG (1 : 50, Vector Laboratories) and then for 2 hours in biotinylated Cy-3 (1 : 500, Vector Laboratories).
Sections were analyzed using a Bio-Rad Radiance Plus confocal scanning microscope (Bio-Rad, Hemel Hempstead, UK), installed on a Nikon Eclipse E600 fluorescence microscope (Nikon, Tokyo, Japan). FITC and Cy3 signals were always detected separately. The FITC labeling was excited using the 488 nm line of the Argon ion laser and detected after passing an HQ513/30 (Bio-Rad) emission filter. For detection of the Cy3 signal, the 543 nm line of the green HeNe laser was used in combination with the 605 / 32 (Bio-Rad) emission filter. Images were imported into Adobe Photoshop ver. 7.0 (Adobe Systems, San Jose, CA, USA) and printed on photo paper (Seiko Epson, Tokyo, Japan). For presentation, all manipulations (brightness and contrast only) were carried out equally for all images.
For whole-mount immunostaining, the same immunocytochemical procedures described above were used, but with a longer incubation time. For semi-quantitative analysis of post-ischemic changes in the NKCC2 immunoreactive ganglion cells, the density (per unit area, 0.25 mm × 0.2 mm) of the labeled ganglion cells was counted in the mid temporal region of the whole-mount retinas. The number of labeled cells was divided by the unit area to obtain mean densities of labeled cells per mm2. Data are given as mean ± standard deviation. Statistical significance was assessed using one-way ANOVA.
To evaluate the postlesional changes in the NKCC2 levels quantitatively, we performed an immunoblot analysis on membranes prepared from control and ischemic rat retina. As shown in Fig. 1, antibodies to the NKCC2 demonstrated a single band with a molecular mass of 100 to 150 kDa, the intensity of which increased up to 3 days following ischemia/reperfusion and gradually declined thereafter, in accordance with the immunocytochemical observations. Densitometric analysis revealed that the NKCC2 level was upregulated to approximately 115% of controls by 1 day and to approximately 415% by 3 days. The NKCC2 levels decreased to 275% and 313% at 7 and 14 days post ischemia/reperfusion, respectively.
The thickness of the inner retina gradually decreased with increasing time after ischemia, in agreement with previous reports [14-16]. In normal rat retina immunostained for the NKCC2, labeling was mainly observed in the ganglion cell layer and outer plexiform layer (Fig. 2). In the ganglion cell layer, axonal staining was stronger than cell body staining. In the outer plexiform layer, labeled profiles were horizontally oriented. After ischemia/reperfusion, the NKCC2 immunoreactivity increased compared with controls.
To identify the nature of the labeled profiles in the ganglion cell and outer plexiform layers, double labeling experiments using antisera against the NKCC2 were performed with anti-Thy1.1 for the ganglion cell layer and anti-calbindin for the outer plexiform layer. In the merged picture (Fig. 3C and 3F), the NKCC2 labeled profiles in the ganglion cell layer also demonstrated Thy1.1 immunoreactivity. Two weeks after injury, the ischemic retina (Fig. 3D-3F) demonstrated a decreased ganglion cell population compared with the normal retina (Fig. 3A-3C). In the other merged picture (Fig. 4C and 4F), the NKCC2 labeled profiles in the inner retina also demonstrated calbindin immunoreactivity. Two weeks after injury, the ischemic retina (Fig. 4D-4F) demonstrated increased horizontal cell process immunoreactivity compared with the normal retina (Fig. 4A-4C).
In the present study, all labeled profiles that lay in the ganglion cell and outer plexiform layers of the control retina and retinae of experimental groups were immunoreactive to antibodies against Thy1.1 and calbindin, respectively. These findings clearly indicate that the NKCC2 is expressed in retinal ganglion and horizontal cells.
The density of the NKCC2-labeled ganglion cells was calculated in unit areas (0.25 mm × 0.2 mm) in the mid-temporal region of the retina (Figs. 5 and 6). In the normal retina, the mean density of labeled ganglion cells was 1255 ± 109 cells per unit area. These values correspond to the previous report of Lafuente et al. [14], Lafuente et al. [15], and Lafuente et al. [16]. The density of labeled cells was altered at 3 days and 2 weeks after reperfusion. The density of the labeled cells was 391 ± 49 (31.2% of normal density) and 185 ± 37 cells/unit area (14.7%) by 3 days and 2 weeks, respectively. These results are in agreement with a prior report by Lafuente et al. [14], Lafuente et al. [15], and Lafuente et al. [16], which demonstrated that retinal ganglion cell loss after retinal ischemia is an ongoing process and that most ganglion cells die after ischemia/reperfusion.
Retinal ganglion cells express a variety of enzymes, neurotransmitter receptors, and voltage-gated ion channels. In circumstances in which these cells are severely stressed, cell injury results. Ca2+ ions are important mediators of cell injury and serve as an ubiquitous intracellular second messenger. Cytosolic Ca2+ ion concentration is maintained at a low concentration compared with extracellular Ca2+ ions. The Ca2+ concentration gradient determines the activity of many enzymes, through activation or inactivation of enzymes such as phospholipases, ATPases, proteases, and endonucleases. Increased intracellular Ca2+ levels induce apoptotic cell death [17-19].
During ischemia, total neuronal cell calcium content may increase to 150% or more compared to controls. Intracellular Ca2+ overloads and activates multiple molecular cascades, which contribute to eventual cell death. Although cell injury results from increased Ca2+ ion concentration, and this in turn mediates irreversible cell death cascades, loss of calcium homeostasis is not always an upstream event. The Ca2+ overload leading to cell death is normally preceded by elevation of intracellular sodium levels, caused by inhibition of the Na+/K+-pump, activation of the Na+/H+ exchange, and Na+-HCO3--cotransport following ATP depletion and acidosis. Reversal of the Na+/Ca2+ exchanger also contributes to intracellular Ca2+ increase. A significant component of axon loss after anoxic injury is due to the reverse operation of the Na+/Ca2+ exchanger triggered by the Na+ influx. The NKCC also contributes to excessive Na+ influx, and persistent Na+ influx accelerates neuronal damage by favoring Ca2+ influx [1-10]. So, theoretically Na+ channel blocking is more effective than calcium channel blocking for preventing these processes [20].
The NKCC is believed to play a critical role in the regulation of extracellular fluid volume and osmolarity. The NKCC1 isoform has been found in a variety of tissues, but the NKCC2 isoform has been identified only in the medullary regions of the kidney. Mutations of the NKCC2 have been reported to be linked to Batter's syndrome, a severe human genetic disease involving hypokalemic alkalosis with hypercalciuria.
In the brain, the NKCC1 maintains sodium concentration under physiological conditions. However, under pathophysiological conditions, such as ischemia, the NKCC1 contributes to an excessive sodium influx, which may induce cell damage via several mechanisms [8]. Mechanisms of acute ischemic injury in developing white matter astrocytes are not associated with Ca2+ influx, but are associated with the NKCC [9]. In ischemic myocardium in diabetics, alteration of the myocardial NKCC permeability results in increased entry of Na+. Inhibition of the NKCC with butmetanide reduced ischemia/reperfusion injury by 50% in diabetic hearts [10]. Such examples suggest key roles of the NKCC in ischemic cellular injury.
In the case of retinal ganglion cells, there have been no reports regarding the existence of the NKCC. As such, there has been no research on the role of the NKCC in retinal ischemia. Previous studies demonstrated that the NKCC is expressed in horizontal cells, starburst amacrine cells, and on bipolar dendritic tips, but not in ganglion cells [12,13]. Only in the developing retina is the NKCC transiently expressed on the ganglion cells. However, these previous studies were performed only in regards to NKCC1; there have been no prior reports on NKCC2 in the retina.
In the normal rat retina, light photomicrographs of vibratome sections demonstrated that NKCC2 labeling cells were observed mainly in the ganglion cell and outer plexiform layers. Axon bundles of ganglion cells are more immunoreactive than cell bodies. Confocal laser scanning microscopy with Thy1.1 and calbindin revealed ganglion cells and horizontal cells as the sources of the NKCC2, respectively. Ganglion and horizontal cell processes have strong NKCC2 immunoreactivity. The NKCC2 was identified as a good marker of retinal ganglion cells, similar to Thy1.1, which has been widely used. Additionally, retinal damage models demonstrated that both protein and mRNA levels of Thy1.1 change to a much greater extent than RGC number loss [21]. As the NKCC2 immunoreactivity increases during ischemia, it can be used as a marker of retinal ganglion cell stress.
Following ischemia/reperfusion, immunoblots of the NKCC2 protein levels increased and reached a peak of approximately 415% of control levels 3 days after ischemia/reperfusion. There is a direct relationship between the duration of insult and the quantity of neuron loss [14]. We raised intraocular pressure to 120 mmHg for 60 minutes. According to prior studies that used a similar experimental paradigm, cellular reaction to ischemic insult has a peak at 3 days [22,23].
As reported in prior research on the quantification of retinal ganglion cell death after 120 minutes of ischemia, 95% of ganglion cells were lost between 5 and 30 days after the insult [24]. The current study demonstrated that the mean density of NKCC2 labeled ganglion cells per unit area (0.25 mm × 0.2 mm) decreased from 1,255 ± 109 to 391 ± 49 (31.2% of normal density) by 3 days and 185 ± 37 (14.7% of normal density) by 2 weeks.
In the current study, although both the ganglion and horizontal cell processes expressed the NKCC2, only the number of ganglion cell decreased; the number of horizontal cells did not decrease. There are several causes for selective cell death of ganglion cells compared with horizontal cells.
N-methyl-D-aspartate (NMDA) toxicity to retinal ganglion cells is well known. As the retinal ganglion cell express NMDA receptors, they are vulnerable to ischemic injury [25]. Inner retinal neurons are also susceptible to ischemic insults as both ionotropic and metabotropic receptors are widely expressed in inner retinal neurons [26]. Because the glutamate receptors expressed on horizontal cells are AMPA receptors [27], they may not have a major role in ischemic cell death. Calcium binding protein is also important; these cells are resistant to ischemia through calcium buffering. Calbindin-labeled horizontal cells are resistant to ischemic insults in the rat retina [28]. Calbindin, an EF-hand calcium binding protein, controls the level of intracellular calcium within the first 100 milliseconds following calcium influx in retinal horizontal cells [29]. Therefore, rat horizontal cells would more resistant to injury due to their greater capacity to buffer intracellular calcium induced by excitotoxicity. Moreover, excessive Ca2+ influx triggers formation of nitric oxide (NO) via neuronal NO synthase (nNOS). In ischemic retina, Muller and retinal ganglion cells express nNOS and inducible NOS. Also, matrix metalloproteinase is activated via neuronal nitric oxide synthase [30,31].
There are few prior reports on horizontal cell changes in ischemia/reperfusion. In the current study, horizontal cell specific immunostaining demonstrated marked axonal sprouting from early phases. Retinal horizontal cells are a famous and long-standing example of gap junctional coupling in all species [32,33]. We assumed that marked axonal sprouting may be due to the gap junction and horizontal cell axonal sprouting may explain the elevated NKCC2 expression. We attempted to investigate the increase of the NKCC2 expression in horizontal cells, but this was not technically feasible as the antibody to the NKCC2 did not penetrate well into the outer plexiform layer.
In summary, we have presented three major findings. First, the NKCC2 is expressed in retinal ganglion and horizontal cells processes. Second, the NKCC2 expression was upregulated in the ischemic rat retina; the NKCC2 immunoreactivity increased after ischemia/reperfusion compared with controls. Third, the number of NKCC2-labeled ganglion cells decreased after retinal ischemia. Our findings lead us to propose that treatment of ischemic insults, such as glaucoma, with inhibitors of the NKCC2 warrants further study.
Figures and Tables
Fig. 1
Immunoblot analysis of the Na+-K+-2Cl--cotransporter 2 (NKCC2) protein levels in normal and ischemic rat retina. (A) Immunoblot stained for the NKCC2 demonstrating a single band at 120 to 130 kDa for all time points tested: normal retina (lane 1), 1 day (lane 2), 3 days (lane 3), 7 days (lane 4), and 14 days (lane 5) after ischemia/reperfusion. (B) Densitometric analysis of immunoblots as shown in (A). The NKCC2 level was upregulated to approximately 115% of control by 1 day and to approximately 415% by 3 days. The NKCC2 levels decreased to 275% and 313% at 7 days and 14 days of ischemia/reperfusion, respectively. Asterisk represents a significant NKCC2 expression increase (p < 0.05, one way ANOVA).

Fig. 2
Immunocytochemical staining for the Na+-K+-2Cl--cotransporter 2 (NKCC2) in normal and ischemic rat retina. Photomicrographs show 50-µm-thick sections processed for immunocytochemistry. (A) Normal retina. (B,C,D,E) Ischemic retina 1, 3, 7, and 14 days after ischemia/reperfusion, respectively. Labeling for the NKCC2 is present in both the ganglion and outer plexiform layers. After ischemia/reperfusion, retinal ganglion cells decrease and marked axonal sprouting in the outer plexiform layer was observed. In the ganglion cell layer, the ganglion cell axon bundles (asterisk) and ganglion cell body (arrow) are seen. Arrow head indicates the horizontal cell processes in the outer plexiform layer. ONL = outer nuclear layer; OPL = outer plexiform layer; INL = inner nuclear layer; IPL = inner plexiform layer; GCL = ganglion cell layer. Scale bar = 50 µm.
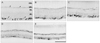
Fig. 3
Confocal micrographs taken from a vertical section of normal retina (A,B,C) and ischemic retina 2 weeks after injury (D,E,F). Tissues were processed for Na+-K+-2Cl--cotransporter 2 (NKCC2) (A,D) and Thy1.1 (B,E) immunohistochemistry. The NKCC2 labeled ganglion cells are immunofluorescent using Cy3-conjugated secondary antibody, and Thy1.1 was visualized using a FITC-conjugated secondary antibody. The NKCC2 demonstrates good immunoreactivity, comparable to Thy1.1 (C,F) Merged picture shows that Thy1.1 immunoreactive profiles show NKCC2 immunoreactivity (yellow). ONL = outer nuclear layer; OPL = outer plexiform layer; INL = inner nuclear layer; IPL = inner plexiform layer; GCL = ganglion cell layer. Scale bar = 50 µm.
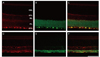
Fig. 4
Confocal micrographs taken from a vertical section of normal retina (A,B,C) and ischemic retina 2 weeks after injury (D,E,F). Tissues were processed for Na+-K+-2Cl--cotransporter 2 (NKCC2) (A,D) and calbindin (B,E) immunohistochemistry. The NKCC2 labeled ganglion cells are immunofluorescent using Cy3-conjugated secondary antibody and calbindin was visualized using a FITC-conjugated secondary antibody. (C,F) Merged picture shows that calbindin immunoreactive profiles show NKCC2 immunoreactivity (yellow). After ischemia/reperfusion, the NKCC2 immunoreactivity increased and the number of horizontal cells did not decrease. ONL = outer nuclear layer; OPL = outer plexiform layer; INL = inner nuclear layer; IPL = inner plexiform layer; GCL = ganglion cell layer. Scale bar = 50 µm.
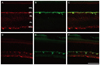
Fig. 5
Light micrographs taken from whole-mount retinas processed for the Na+-K+-2Cl--cotransporter 2 (NKCC2) in normal retina (A) and 3 (B) and 7 days (C) after ischemia/reperfusion. The focus is at the ganglion cell layer. In normal retina, the NKCC2 labeled ganglion cells (arrows) and axon bundles are clearly distinguishable. In the ischemic retina, the number of the NKCC2 labeled ganglion cells decreased compared with the normal retina. Scale bar = 50 µm.

Fig. 6
Densities of the Na+-K+-2Cl--cotransporter 2 (NKCC2) immunoresponsive retinal ganglion cells after transient ischemia/reperfusion (cells/mm2). Densities decreased significantly 3 days and 2 weeks after injury. Asterisk represents a significant decrease of the NKCC2-labeled ganglion cell densities (p < 0.05, one way ANOVA).

References
1. Adorante JS, Miller SS. Potassium-dependent volume regulation in retinal pigment epithelium is mediated by Na,K,Cl cotransport. J Gen Physiol. 1990. 96:1153–1176.
2. Russell JM. Sodium-potassium-chloride cotransport. Physiol Rev. 2000. 80:211–276.
3. Haas M, Forbush B 3rd. The Na-K-Cl cotransporter of secretory epithelia. Annu Rev Physiol. 2000. 62:515–534.
4. Leung S, O'Donnell ME, Martinez A, Palfrey HC. Regulation by nerve growth factor and protein phosphorylation of Na/K/2Cl cotransport and cell volume in PC12 cells. J Biol Chem. 1994. 269:10581–10589.
5. Iwamoto LM, Fujiwara N, Nakamura KT, Wada RK. Na-K-2Cl cotransporter inhibition impairs human lung cellular proliferation. Am J Physiol Lung Cell Mol Physiol. 2004. 287:L510–L514.
6. Hains BC, Waxman SG. Neuroprotection by sodium channel blockade with phenytoin in an experimental model of glaucoma. Invest Ophthalmol Vis Sci. 2005. 46:4164–4169.
7. Kim JA, Kang YY, Lee YS. Activation of Na(+), K(+), Cl(-)-cotransport mediates intracellular Ca(2+) increase and apoptosis induced by Pinacidil in HepG2 human hepatoblastoma cells. Biochem Biophys Res Commun. 2001. 281:511–519.
8. Chen H, Sun D. The role of Na-K-Cl co-transporter in cerebral ischemia. Neurol Res. 2005. 27:280–286.
9. Thomas R, Salter MG, Wilke S, et al. Acute ischemic injury of astrocytes is mediated by Na-K-Cl cotransport and not Ca2+ influx at a key point in white matter development. J Neuropathol Exp Neurol. 2004. 63:856–871.
10. Ramasamy R, Payne JA, Whang J, et al. Protection of ischemic myocardium in diabetics by inhibition of electroneutral Na+-K+-2Cl- cotransporter. Am J Physiol Heart Circ Physiol. 2001. 281:H515–H522.
11. Rothman SM. The neurotoxicity of excitatory amino acids is produced by passive chloride influx. J Neurosci. 1985. 5:1483–1489.
12. Fischer KF, Lukasiewicz PD, Wong RO. Age-dependent and cell class-specific modulation of retinal ganglion cell bursting activity by GABA. J Neurosci. 1998. 18:3767–3778.
13. Vardi N, Zhang LL, Payne JA, Sterling P. Evidence that different cation chloride cotransporters in retinal neurons allow opposite responses to GABA. J Neurosci. 2000. 20:7657–7663.
14. Lafuente MP, Villegas-Perez MP, Selles-Navarro I, et al. Retinal ganglion cell death after acute retinal ischemia is an ongoing process whose severity and duration depends on the duration of the insult. Neuroscience. 2002. 109:157–168.
15. Lafuente MP, Villegas-Perez MP, Sobrado-Calvo P, et al. Neuroprotective effects of alpha(2)-selective adrenergic agonists against ischemia-induced retinal ganglion cell death. Invest Ophthalmol Vis Sci. 2001. 42:2074–2084.
16. Lafuente MP, Villegas-Perez MP, Mayor S, et al. Neuroprotective effects of brimonidine against transient ischemia-induced retinal ganglion cell death: a dose response in vivo study. Exp Eye Res. 2002. 74:181–189.
17. Sucher NJ, Lipton SA, Dreyer EB. Molecular basis of glutamate toxicity in retinal ganglion cells. Vision Res. 1997. 37:3483–3493.
18. Osborne NN, Ugarte M, Chao M, et al. Neuroprotection in relation to retinal ischemia and relevance to glaucoma. Surv Ophthalmol. 1999. 43:Suppl 1. S102–S128.
19. Osborne NN, Wood JP, Chidlow G. Invited review: neuroprotective properties of certain beta-adrenoceptor antagonists used for the treatment of glaucoma. J Ocul Pharmacol Ther. 2005. 21:175–181.
20. Dong CJ, Hare WA. Contribution to ischemic injury of rat optic nerves by intracellular sodium overload. Doc Ophthalmol. 2005. 110:15–23.
21. Huang W, Fileta J, Guo Y, Grosskreutz CL. Downregulation of Thy1 in retinal ganglion cells in experimental glaucoma. Curr Eye Res. 2006. 31:265–271.
22. Gwon JS, Kim IB, Lee MY, et al. Expression of clusterin in Müller cells of the rat retina after pressure-induced ischemia. Glia. 2004. 47:35–45.
23. Dijk F, van Leeuwen S, Kamphuis W. Differential effects of ischemia/reperfusion on amacrine cell subtype-specific transcript levels in the rat retina. Brain Res. 2004. 1026:194–204.
24. Selles-Navarro I, Villegas-Perez MP, Salvador-Silva M, et al. Retinal ganglion cell death after different transient periods of pressure-induced ischemia and survival intervals. A quantitative in vivo study. Invest Ophthalmol Vis Sci. 1996. 37:2002–2014.
25. Siliprandi R, Canella R, Carmignoto G, et al. N-methyl-D-aspartate-induced neurotoxicity in the adult rat retina. Vis Neurosci. 1992. 8:567–573.
26. Buchi ER, Suivaizdis I, Fu J. Pressure-induced retinal ischemia in rats: an experimental model for quantitative study. Ophthalmologica. 1991. 203:138–147.
27. Huang SY, Liang PJ. Ca2+-permeable and Ca2+-impermeable AMPA receptors coexist on horizontal cells. Neuroreport. 2005. 16:263–266.
28. Chun MH, Kim IB, Ju WK, et al. Horizontal cells of the rat retina are resistant to degenerative processes induced by ischemia-reperfusion. Neurosci Lett. 1999. 260:125–128.
29. Wassle H, Peichl L, Airaksinen MS, Meyer M. Calcium-binding proteins in the retina of a calbindin-null mutant mouse. Cell Tissue Res. 1998. 292:211–218.
30. Kobayashi M, Kuroiwa T, Shimokawa R, et al. Nitric oxide synthase expression in ischemic rat retinas. Jpn J Ophthalmol. 2000. 44:235–244.
31. Manabe S, Gu Z, Lipton SA. Activation of matrix metalloproteinase-9 via neuronal nitric oxide synthase contributes to NMDA-induced retinal ganglion cell death. Invest Ophthalmol Vis Sci. 2005. 46:4747–4753.
32. Massey SC, O'Brien JJ, Trexler EB, et al. Multiple neuronal connexins in the mammalian retina. Cell Commun Adhes. 2003. 10:425–430.
33. Garcia-Dorado D, Ruiz-Meana M, Padilla F, et al. Gap junction-mediated intercellular communication in ischemic preconditioning. Cardiovasc Res. 2002. 55:456–465.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download