Abstract
Purpose
To investigate the clinical characteristics of polypoidal choroidal vasculopathy (PCV) associated with chronic central serous chorioretinopathy (CSC).
Methods
We retrospectively reviewed the medical records of 246 PCV patients (283 eyes) between July 2004 and August 2009 and investigated the clinical characteristics of the PCV patients who had specific fundus findings of chronic CSC.
Results
Among PCV patients, 13 eyes (4.6%) of 13 PCV patients (5.3%) had fundus findings of chronic CSC. All of the PCV lesions had a solitary polyp located outside the atrophic retina, predominantly in the macular area (84.6%), most showed an exudative pattern (69.2%) and there were a few that showed a hemorrhagic pattern (30.8%). All of the lesions were smaller than 1 disc diameter. Most of the PCV lesions (76.9%) were cured with less than two treatments in a short period of 6.4 ± 1.9 months; however, visual acuity deteriorated (61.5%) or was not changed (30.8%) in most of the cases.
Conclusions
The PCV associated with chronic CSC had several clinical features such as a small exudative retinal lesion with a solitary polyp and frequent involvement of the macular area. Even though there was poor visual outcome due to the atrophic change, all of the PCV lesions were easily resolved in a short period with a simple treatment course and no recurrence.
Typical central serous chorioretinopathy (CSC) is characterized by acute-onset serous retinal detachment as well as a benign visual prognosis with the self-resolution of retinal detachment after several months. Chronic CSC, which is defined as a persistent serous retinal detachment or recurrent multifocal detachments, may lead to diffuse decompensation of the retinal pigment epithelium (RPE), gradual multifocal RPE leakage with fluorescein angiography (FAG), and finally a poor visual outcome due to the atrophic retina and RPE [1-10].
Chronic CSC, which is also known as diffuse retinal pigment epitheliopathy, shows specific retinal changes such as multiple RPE atrophies, RPE hyperpigmentations, and gravitational atrophic RPE tracts. As the indocyanine green angiography (ICGA) of the CSC demonstrated several choroidal findings such as choroidal hyperpermeability, choroidal lobular ischemia and choroidal venous congestion, it is accepted that the pathogenesis of CSC is associated with the dysfunction of choroidal vessels [9-15]. Polypoidal choroidal vasculopathy (PCV) is characterized by a branching vascular network (BVN) with terminal polypoidal dilations. Many studies on PCV have been conducted in Asian countries since the incidence of PCV is relatively high in Asians [16-18]. PCV is reported to originate from inner choroidal vessel abnormalities similar to that of CSC, and PCV also has a specific ICGA finding of CSC, which is known as choroidal hyperpermeability. It thus appears that the pathogeneses of these two diseases have some common features [19,20]. A history of CSC was actually found to be more prevalent in PCV in a study of the relationship between a past history of CSC and the onset incidences of PCV or typical age-related macular degeneration (AMD). Moreover, specific funduscopic findings of CSC, like atrophic RPE tract or focal photocoagulation scars, were found more frequently in PCV [21].
We retrospectively review the medical records of patients with PCV associated with chronic CSC and investigated the clinical characteristic of these PCV patients.
We retrospectively reviewed the medical records of 246 PCV patients, for a total of 283 eyes, who had visited our clinic (Kyungpook National University Hospital) between July 2004 and August 2009. We extracted the PCV patients that had specific fundus findings of chronic CSC and then investigated the clinical characteristics of these PCV patients. All of the PCV patients underwent a comprehensive ophthalmic examination, that included Snellen visual acuity, biomicroscopy, fundus photography, FAG, ICGA (HRA system; Heidelberg Engineering, Heidelberg, Germany), and optical coherence tomography (OCT; Stratus OCT or Cirrus OCT, Carl Zeiss Meditec, Dublin, CA, USA).
Chronic CSC was objectively diagnosed based on the presence of specific fundus findings like an atrophic RPE tract, multiple RPE atrophies and an FAG finding showing hypofluorescence due to a window defect or partial hyperfluorescence due to a leak from diffuse RPE decompensation. We confirmed an atrophic lesion as chronic CSC with the exclusion of AMD or PCV with ICGA in cases with a small leakage area of FAG.
The clinical diagnosis of PCV in older patients was made on the basis of the fundus and ICGA findings. The hemorrhagic or exudative lesion was typically diagnosed as PCV based on the presence of the BVN with single or multiple polyp-like terminal dilations in ICGA and an elevated red-orange finding in the fundus. The diagnosis of PCV also included the presence of polyp-like terminal dilations, which was a distinctive finding of PCV, without identifiable BVN or an elevated red-orange lesion. All of the patients were interviewed on their histories of systemic disease such as hypertension, diabetes mellitus or other retinal diseases that were also evaluated in the other eye of the patient.
Fundus findings were classified as one of two patterns, either as an exudative pattern or a hemorrhagic pattern. In the exudative pattern, serous pigment epithelium detachment (PED) and/or retinal detachment was predominant with little or no hemorrhage. In the hemorrhagic pattern, the hemorrhagic PED and/or submacular hemorrhage were predominant in the lesion. The polyp location and the polyp configuration were identified with ICGA. The polyp location was evaluated on the basis of the locations of the disc and fovea. There were two types of polyp configurations, the solitary type and the cluster type that is composed of more than two polyps [22]. We also evaluated the polyp location compared to the location of the atrophic retina of chronic CSC and whether the polyps were involved in a single area or in multiple areas of the fundus. The number and method of treatments as well as when the lesion state reached a stable condition after treatment were also investigated.
Among 283 eyes of 246 PCV patients, 13 eyes (4.6%) of 13 patients (5.3%) (right eye, 9 [69.2%]; left eye, 4 [30.8%]) had specific fundus findings of chronic CSC (Table 1). The mean age of PCV onset was 69.8 ± 6.2 years (range, 55 to 79 years), and 11 (84.6%) of the patients were men. The overall duration of follow-up after PCV onset was 27.0 ± 18.4 months (range, 6 to 64 months). The resolution of the PCV lesion took 6.4 ± 1.9 months (range, 4 to 10 months) through various treatments after PCV onset, and there were no recurrences after the resolution of PCV for 20.0 ± 17.7 months (range, 1 to 55 months). Of these 13 PCV patients, three had hypertension and none of them had diabetes. All of the patients were unilaterally affected with PCV, but 11 (84.6%) of the 13 patients also had specific fundus findings of chronic CSC in their fellow eyes.
Eleven polypoidal lesions (84.6%) in ICGA were located in the macular area, which were all within 0.5 disc diameters (DD) from the fovea (Fig. 1), and two of the lesions (15.4%) were in the peripapillary area, within 0.5 DD from the margin of the optic disc (Fig. 2). The exudative pattern was found in the fundus in nine of the patients (69.2%), and the hemorrhagic pattern was found in four of the patients (30.8%). Among the four eyes with the hemorrhagic pattern, only two (15.4%) had a definite hemorrhagic PED. Most of the lesions in the other two eyes were composed of exudative lesions, and there was only a small amount of retinal hemorrhage in the larger area of exudative lesion. The sizes of all of the lesions, regardless of their pattern, were less than 1 DD in area, and none of the lesions had extensive hemorrhage or exudation. All 13 eyes had a solitary polyp configuration. A prominent BVN could not be found near any of the polyps, even though large choroidal vessels were easily found in the atrophic area around the polyp in all of the eyes. The ICGA findings showed that three of 13 patients had polyps that were involved in multiple areas of the fundus, and the other 10 patients (76.9%) had a single area of PCV involvement. In regard to the location of a polyp compared to the atrophic retinal area, there were no polyps that were identified within the atrophic retina of the chronic CSC lesion or distant from the atrophic retina. All of the polyps were located around the boundary of the atrophic retina and were identified within 0.5 DD of the normal retina from the boundary of the atrophic retina.
Various treatments such as intravitreal ranibizumab injection, intravitreal bevacizumab injection, photodynamic therapy, and conventional laser photocoagulation were conducted. Including the one patient whose retinal detachment was absorbed without treatment, the average number of treatments was 1.53 (0 to 4), and 10 cases had complete resolution within two treatments (76.9%). The mean best-corrected visual acuity (BCVA) at presentation of PCV was 0.47 ± 0.24 (0.16 to 1) with the conversion to logarithm of the minimum angle of resolution, and the mean final BCVA after the complete resolution of the PCV lesion was 0.71 ± 0.39 (0 to 1.3). All of the PCV lesions were cured into the atrophic state without fibrotic scar tissue.
The abnormalities of inner choroidal vessels have been introduced as the primary pathogenesis of both CSC and PCV in a number of studies. In the ICGA studies of CSC, a diffuse choroidal hyperpermeability was considered as a main pathogenesis of CSC. In the pathogenesis model of CSC, diffuse choroidal hyperpermeability causes blister elevations (serous RPE detachments). Increasing choroidal pressure mechanically induces RPE tearing or RPE decompensation, which finally results in RPE leakage into the subretinal space and a neurosensory detachment (serous retinal detachment) [1-8]. This similar pathogenesis was also proposed in the pathologic finding of PCV. In a recent study of PCV pathology, the primary pathologic finding of PCV was reported as dilated hyalinized choroidal vessels followed by a massive extravasation of plasma protein into the choroidal tissue without granulation. This also included tissue proliferation, which is also found in choroidal neovascularization, and massive exudation induced by abnormal choroidal vesssels can sufficiently increase the choroidal tissue pressure to produce the protrusion of choroidal tissues through the weak area of RPE and Bruch's membrane. In this study, arteriosclerotic changes of the choroidal vessels were proposed as the main pathogenesis of these pathologic findings [23].
The mutual correlation of pathogenesis between PCV and CSC has been proposed [24], which suggests the possibility that the RPE changes in chronic CSC might predispose one to polypoidal changes of choroidal vessels. Another PCV study reported that the choroidal vascular hyperpemeability, which was the characteristic ICGA finding of CSC, was more prevalent in PCV than in typical AMD (9.8% vs. 1.9%), and other ICGA findings of CSC, such as choroidal filling delay and a dilated choroidal vein, were more common in PCV as well [20]. The direct correlation between PCV onset and a history of CSC was recently investigated [21]. In a study of PCV in Japan, which compared the background factor of PCV and typical AMD, a past history of CSC was more frequently found in PCV patients than in AMD patients (14.7% vs. 3.4%). In a study on objective fundus findings that more likely suggested a history of CSC, an atrophic RPE tract and focal photocoagulation scars were more frequently found in PCV patients than in AMD patients (7.6% vs. 0.7%).
We also investigated FAG, ICGA and fundus findings, such as the RPE atrophic tract, and multiple RPE atrophies in order to objectively study the clinical findings of PCV associated with chronic CSC. Among 283 eyes of 246 PCV patients, PCV onset with specific fundus findings of chronic CSC occurred in 13 eyes (4.6%) of 13 patients (5.3%). This rate was similar to that reported in the Ueta study, which disclosed that 11 patients (6.5%) had a RPE atrophic scar without photocoagulation scars of 170 PCV patients [21]. In the current study, many patients (11 patients, 84.6%) also had fundus findings of chronic CSC in their other eye, but none of the patients had PCV lesions in both eyes.
PCV lesions were most frequently located in the macular area in the PCV studies of Koreans. One PCV study in Korea reported that the PCV lesions in ICGA were located in the macula (87.8%), in the peripapillary area (5%), and in both macula and peripapillary area (7.2%). Another PCV study reported that these types of lesions were located in the macula (62.5%), in the peripapillary area (7.5%), and in both macula and peripapillary area (6.3%) [17,25]. Also, the macular area was most frequently involved with the PCV lesion (84.6%) in the current study.
When we investigated the polyp location in relation to the atrophic retina of chronic CSC, all of the polyps were outside the boundary of the atrophic retina. There was no polypoidal appearance within the atrophic retina. We postulated that, in a severely atrophied retina, a polyp cannot be produced due to severe atrophy of all of the chorodial vessels, including the choriocapillaris and the choroidal artery/vein, which would cause them to lose their ability to dilate and protrude. It is believed that the reason why polyps were not found in the distant area was due to the fact that the distant retina would have little risk of PCV occurrence since this area would contain less damaged choroidal vessels, which were less influenced by the chronic CSC due to the far distance.
In the current study, the PCV lesion had a small sized lesion of less than 1 DD in area that did not have either extensive hemorrhage or exudation. Most of the patients had the exudative pattern (9 eyes, 69.2%), and two of the four patients with the hemorrhagic pattern had larger exudative lesions than hemorrhage lesions in their PCV lesions. In regard to the polyp configuration of the current study, all of the polyps were of the solitary type even in the cases that had polyps that were identified in multiple areas of the ICGA. In contrast to the current study, previous PCV studies on Koreans reported that cases with a hemorrhagic pattern and cluster type polyps were more prevalent than cases with the exudative pattern. One report showed that the hemorrhagic pattern occurred in 61.3% of cases, which included an extensive hemorrhage, and cluster type polyps accounted for 74.5%, which included polyps with a microaneurysm and a large aneurysm, and another study showed that subretinal hemorrhage occurred in 63.8% of cases and cluster type polyps occurred in 60% of cases [17,25]. This difference between the fundus findings of the current study and of previous studies may have been due to the generalized atrophied choroidal tissue, including the vessels, not leading to active PCV lesions as in an extensive BVN or a cluster of polyps, even though the predisposition of PCV occurrence would increase due to the choroidal vascular abnormality of the chronic CSC.
A study on the natural history of PCV has reported that a solitary polyp that is usually stable with favorable clinical course will tend to regress and disappear, and that a cluster of polyps in an active lesion, which has a high risk factor indicative of hemorrhage and leakage, had a worse prognosis. They also reported that the hemorrhagic pattern had repeated recurrences of acute bleeding that caused a sudden decrease in visual acuity, and that the exudative pattern that is associated with intraretinal lipid deposits maintained a similar exudation for a long period of time, which was then followed by degeneration and atrophy of the RPE that then led to the decrease in visual acuity [22]. Similar to a previous study, all of the PCV lesions in the current study had a solitary polyp that maintained a stable state without active bleeding or recurrence, and most of the PCV lesions had the exudative pattern that produced an atrophic retina and choroid. Actually, all of the lesions in our study were more atrophied after the resolution of the PCV lesion even in those with the hemorrhagic pattern, which was partially due to the generalized atrophic retinal state due to the chronic CSC.
To the complete resolution of the PCV lesion after treatment, the follow-up period was slightly short at 6.4 ± 1.9 months (4 to 10 months), and in 10 of the cases (76.9%), including one self-resolution case, the PCV lesions were cured with less than two courses of treatment. None of the PCV lesions in the current study required surgery for massive hemorrhage or a complicated course of treatments; we assumed that this was because the lesions had an inactive character like a solitary polyp, a small sized exudative lesion or a generalized atrophic retina without highly active lesions like a cluster of polyps or a large BVN.
However, there was no change in four eyes (30.8%) in regard to the visual acuity, and eight eyes (61.5%) worsened in visual acuity, which means that this was more common an occurrence than an improvement in visual acuity, which only occurred in one eye (7.7%). We believed the worsened visual acuity was induced by the atrophic damage from the PCV lesion in the macular area, which was the most common site of the PCV occurrence (84.6%).
A benefit of the current study is that it used more objective fundus findings, which are more likely to suggest a history of chronic CSC, but also has the limitation in that the study could not investigate the longstanding disease course from the onset of typical acute CSC. Other limitations of this study are the small number of patients enrolled and the short follow-up time of some of the cases.
In conclusion, the PCV lesion associated with chronic CSC has several clinical characteristics. Most of the PCV lesions had a solitary polyp, which was found in multiple lesions in some of the cases, and there were no cluster of polyps or large BVN observed. The exudative retinal lesion with little or no hemorrhage was the most common retinal finding, and the macular area was the most common site of PCV occurrence. All of the polyps were located within 0.5 DD outside the boundary of the atrophic lesion. Visual acuities in most of the cases were deteriorated, and we guessed this was due to the atrophic damage caused by the PCV lesion occurring in the macular area. However, there was no need for the multiple and complicated treatment courses of these patients, and all of the PCV lesions were easily resolved to an atrophic state within a short period of time and maintained a stable state without recurrence throughout the follow-up period.
References
1. Hayashi K, Hasegawa Y, Tokoro T. Indocyanine green angiography of central serous chorioretinopathy. Int Ophthalmol. 1986; 9:37–41. PMID: 3721709.

2. Scheider A, Nasemann JE, Lund OE. Fluorescein and indocyanine green angiographies of central serous choroidopathy by scanning laser ophthalmoscopy. Am J Ophthalmol. 1993; 115:50–56. PMID: 8420378.

3. Piccolino FC, Borgia L. Central serous chorioretinopathy and indocyanine green angiography. Retina. 1994; 14:231–242. PMID: 7973118.

4. Guyer DR, Yannuzzi LA, Slakter JS, et al. Digital indocyanine green videoangiography of central serous chorioretinopathy. Arch Ophthalmol. 1994; 112:1057–1062. PMID: 8053819.

5. Piccolino FC, Borgia L, Zinicola E, Zingirian M. Indocyanine green angiographic findings in central serous chorioretinopathy. Eye (Lond). 1995; 9(Pt 3):324–332. PMID: 7556741.

6. Spaide RF, Hall L, Haas A, et al. Indocyanine green videoangiography of older patients with central serous chorioretinopathy. Retina. 1996; 16:203–213. PMID: 8789858.

7. Prünte C, Flammer J. Choroidal capillary and venous congestion in central serous chorioretinopathy. Am J Ophthalmol. 1996; 121:26–34. PMID: 8554078.
8. Menchini U, Virgili G, Lanzetta P, Ferrari E. Indocyanine green angiography in central serous chorioretinopathy. ICG angiography in CSC. Int Ophthalmol. 1997; 21:57–69. PMID: 9405986.
9. Yannuzzi LA, Shakin JL, Fisher YL, Altomonte MA. Peripheral retinal detachments and retinal pigment epithelial atrophic tracts secondary to central serous pigment epitheliopathy. Ophthalmology. 1984; 91:1554–1572. PMID: 6084221.

10. Yannuzzi LA, Slakter JS, Kaufman SR, Gupta K. Laser treatment of diffuse retinal pigment epitheliopathy. Eur J Ophthalmol. 1992; 2:103–114. PMID: 1450655.

11. Castro-Correia J, Coutinho MF, Rosas V, Maia J. Long-term follow-up of central serous retinopathy in 150 patients. Doc Ophthalmol. 1992; 81:379–386. PMID: 1486812.

12. Spaide RF, Campeas L, Haas A, et al. Central serous chorioretinopathy in younger and older adults. Ophthalmology. 1996; 103:2070–2079. PMID: 9003341.

13. Spraul CW, Lang GE, Lang GK. Retinal pigment epithelial changes associated with systemic corticosteroid treatment: report of cases and review of the literature. Ophthalmologica. 1998; 212:142–148. PMID: 9486556.

14. Bujarborua D. Long-term follow-up of idiopathic central serous chorioretinopathy without laser. Acta Ophthalmol Scand. 2001; 79:417–421. PMID: 11453866.

15. Otsuka S, Ohba N, Nakao K. A long-term follow-up study of severe variant of central serous chorioretinopathy. Retina. 2002; 22:25–32. PMID: 11884874.

16. Maruko I, Iida T, Saito M, et al. Clinical characteristics of exudative age-related macular degeneration in Japanese patients. Am J Ophthalmol. 2007; 144:15–22. PMID: 17509509.

17. Byeon SH, Lee SC, Oh HS, et al. Incidence and clinical patterns of polypoidal choroidal vasculopathy in Korean patients. Jpn J Ophthalmol. 2008; 52:57–62. PMID: 18369702.

18. Wen F, Chen C, Wu D, Li H. Polypoidal choroidal vasculopathy in elderly Chinese patients. Graefes Arch Clin Exp Ophthalmol. 2004; 242:625–629. PMID: 15257461.

19. Yuzawa M, Mori R, Kawamura A. The origins of polypoidal choroidal vasculopathy. Br J Ophthalmol. 2005; 89:602–607. PMID: 15834093.

20. Sasahara M, Tsujikawa A, Musashi K, et al. Polypoidal choroidal vasculopathy with choroidal vascular hyperpermeability. Am J Ophthalmol. 2006; 142:601–607. PMID: 17011852.

21. Ueta T, Obata R, Inoue Y, et al. Background comparison of typical age-related macular degeneration and polypoidal choroidal vasculopathy in Japanese patients. Ophthalmology. 2009; 116:2400–2406. PMID: 19815291.

22. Uyama M, Wada M, Nagai Y, et al. Polypoidal choroidal vasculopathy: natural history. Am J Ophthalmol. 2002; 133:639–648. PMID: 11992861.
23. Nakashizuka H, Mitsumata M, Okisaka S, et al. Clinicopathologic findings in polypoidal choroidal vasculopathy. Invest Ophthalmol Vis Sci. 2008; 49:4729–4737. PMID: 18586873.

24. Ahuja RM, Downes SM, Stanga PE, et al. Polypoidal choroidal vasculopathy and central serous chorioretinopathy. Ophthalmology. 2001; 108:1009–1010. PMID: 11382610.

25. Lee JW, Kim IT. Epidemiologic and clinical characteristics of polypoidal choroidal vasculopathy in Korean patients. J Korean Ophthalmol Soc. 2007; 48:63–74.
Fig. 1
A 73-year-old man (case 12) with a polypoidal choroidal vasculopathy lesion in the submacular hemorrhagic lesion. (A) In fundus photography, an atrophic retinal pigment epithelium (RPE) tract and a submacular hemorrhage were found in the macular area and (B,C) a fluorescein angiography and an indocyanine green angiography (ICGA) showed a solitary polyp near the atrophic RPE tract. (D) Optical coherence tomography showed pigment epithelial detachment in the polypoidal lesion of ICGA, which was scanned on the white line of (C).
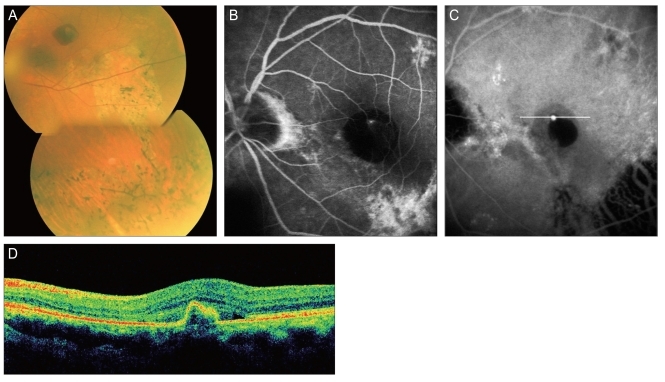
Fig. 2
A 66-year-old man (case 7) with a polypoidal choroidal vasculopathy lesion around the peripapillary area. (A) Fluorescein angiography (FAG) showed atrophic lesions corresponding to an atrophic retinal pigment epithelium tract and the atrophic lesion in the superior peripapillary area. The polypoidal lesion was found near the superior atrophic lesion. (B) indocyanine green angiography (ICGA) also showed a solitary polyp in the polypoidal lesion of the FAG. (C) Optical coherence tomography showed the pigment epithelium detachment (white arrow) in the polypoidal lesion of the ICGA, which was scanned on the white line of (B).
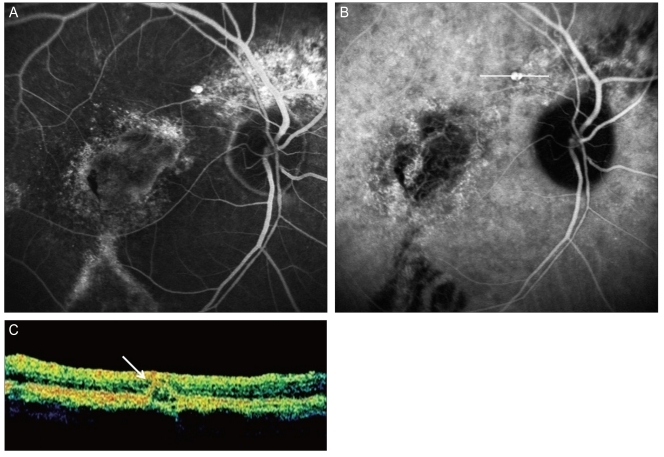
Table 1
Clinical data of patients with polypoidal choroidal vasculopathy
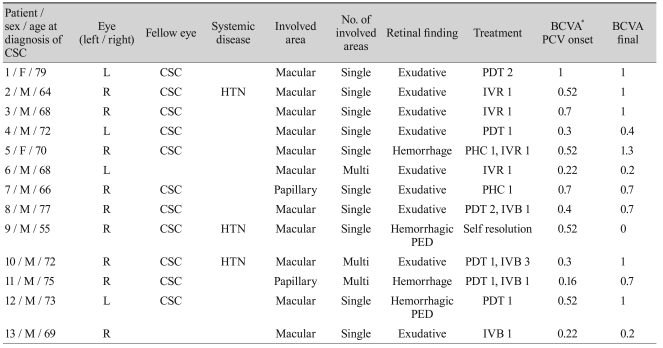
CSC = central serous chorioretinopathy; BCVA = best-corrected visual acuity; PCV = polypoidal choroidal vasculopathy; HTN = hypertension; PDT = photodynamic therapy; IVR = intravitreal ranibizumab injection; IVB = intravitreal bevacizumab injection; PHC = conventional laser photocoagulation; PED = pigment epithelium detachment.
*BCVA in logarithm of the minimum angle of resolution acuity.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download