Abstract
Purpose
To investigate the effect of dark rearing immediately after birth on the maturation of the visual relay neurons in the lateral geniculate nucleus.
Methods
Fifty neonatal rats were used. Neonates of the control groups were raised under a normal light/dark cycle. Neonates of the experiment groups were dark reared and isolated from light during the entire experimental period, then exposed to the sun light for 1 hour before sacrifice.
Results
In the control groups, the neurons in the dorsal lateral geniculate nucleus developed normally at each age tested. In the experiment groups, the cytoplasm of the large neurons in the dorsal lateral geniculate nucleus of 2-week-old rats contained small vesicles, and the cytoplasm of the large neurons of 4-week-old rats was converted into a vacuole-like space. Moreover, c-Fos immunoreactivity of the large neurons in the dorsal lateral geniculate nucleus in the experiment groups was significantly increased compared to that of the control groups.
Conclusions
We suppose that the maturation of the neurons in the lateral geniculate nucleus might be influenced by light stimulation during the critical period. Furthermore, c-Fos could be a marker of the functional activity of the visual relay neurons of the lateral geniculate nucleus in albino rats.
In mammals, the visual cells in the retina obtain visual information by perceiving light and then processing it as neural signals. The nerve fibers from the retina run through the optic nerve and the optic chiasm forming the optic tract and are transmitted to the visual relay neurons in the lateral geniculate nucleus (LGN). The axons of the visual relay neurons in the LGN transmit information to the visual cortex through the optic radiations. The genes expressed in the neurons in response to visual stimulation are classified as immediate early genes (IEGs) or late response genes, according to their expression time after stimulation [1]. It is known that IEGs are activated temporarily and rapidly in response to diverse cellular stimulations. Currently, c-Fos, c-myc, and c-jun are the most widely known IEGs. Previous studies have reported that the expression of the c-Fos gene was induced rapidly and temporarily within a few minutes in response to external stimulation [2-4]. Furthermore, c-Fos protein translated by the c-Fos gene is able to induce the transcription factors that can activate or suppress the expressions of specific genes [5]. In the visual transmission system such as the retina, the superior colliculus, and the visual cortex, expression of the c-Fos gene related to light stimulation has been reported [6-8]. Nonetheless, few studies on the expression of the c-Fos gene within the LGN have been performed. Therefore, we examined both the morphological changes and the expression of c-Fos immunoreactivity in the LGN induced by light exposure.
For the experiments, albino rats (Sprague-Dawley strain) weighing approximately 250 grams were used. Ten neonatal albino sibling rats were divided immediately after birth into the control group (4 rats) and experimental group (6 rats). In total, five pregnant albino rats (50 neonates) were used in the same way. Therefore, the control groups contained 20 neonates, and the experiment groups contained 30 neonates. In order to manage the albino rats, we adhered strictly to the Guiding Principles in the Care and Use of Animals (US Department of Health, Education and Welfare publication NIH 85-23).
In the control group, each scheduled period (I. immediately after birth, II. 1 week after birth, III. 2 weeks after birth, IV. 4 weeks after birth) contained 5 neonates. As scheduled, regardless of their gender, aspiration anesthesia with ethyl ether was induced between 10 o'clock and 12 o'clock in the morning. The chest was then opened, and the perfusion fixation was performed with 4% paraformaldehyde-0.1% glutaraldehyde solution through the left ventricle of the neonatal albino rats up to 2 weeks after birth and through the ascending aorta of the neonatal albino rats 4 weeks after birth. The brain was then extracted, and post-fixation was performed for 24 hours using the same solution as in the perfusion-fixation.
In the experiment group, immediately after birth, neonatal albino rats were reared in a dark room for the scheduled periods (I. 1 week after birth, II. 2 weeks, III. 4 weeks). Each stage contained 10 neonates. Immediately after the scheduled dark-rearing, regardless of their gender, the rats were exposed to sunlight for 1 hour and anesthetized. The perfusion fixation and the post-fixation were then performed using the same methods as in the control group.
For the preparation of samples for microscope analysis, paraffin sections 7 µm thick were made. The samples were washed with acidic alcohol and attached to the glass slides with surfaces that were pretreated with gelatin, and paraffin sections 14 µm thick were made and attached to SuperFrost plus glass slides (Fisher Scientific Co., Pittsburgh, PA, USA).
Morphological examination was conducted after deparaffinization using xylene and hydration with different concentrations of alcohol, hematoxylin and eosin staining was performed to examine the general morphological changes of the brain, and luxol fast blue as well as cresyl violet staining (Kluver-Barrera method) were performed to examine the myelinated nerve fibers.
For immunohistochemistry using the c-Fos protein antibody, the prepared samples were treated using the identical method of the deparaffinization and hydration process, washed three times with 0.05 M Tris buffer adjusted to pH 7.6 (Tris buffered saline, TBS), and fixed for 10 minutes using 4% paraformaldehyde-0.1% glutaraldehyde. In order to suppress the activation of endogenous hydrogen peroxide, the samples were immersed in a 3% H2O2-methanol mixture solution for 30 minutes and again washed three times with 0.05 M TBS for 5 minutes. To restore the antigenicity of the c-Fos protein, under the condition that the sample slides were immersed completely in 0.05 M citrate buffer solution and adjusted to pH 7.6, the samples were heated in a microwave at 100℃ for 10 minutes and cooled at room temperature. To suppress non-specific reactions, the samples were washed for 15 minutes with 0.05 M TBS-A, 0.5% triton X-100, and 0.05 M TBS-B, where 5% goat serum was added to TBS-A.
As the primary antibody, the anti-c-Fos antibody (rabbit-polyclonal; Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) diluted to 1:100 with 0.05 M TBS-B was used, reacted at 4℃ for 72 hours, and washed with TBS-A and TBS-B for 15 minutes each. For the secondary antibody, biotinylated goat anti-rabbit IgG (Vector Co., Burlingame, CA, USA) diluted to 1:200 with TBS-B was used, reacted at room temperature for 1 hour, washed with TBS-A and TBS-B for 15 minutes each, and reacted for 1 hour with avidin-biotin-peroxidase complex (ABC method) (Vectastain Elite ABC kit, Vector Co.) at room temperature.
As a dye, 0.33% 3.3-diaminobenzidine tetrahydrochloride (10 mg/40 mL TBS; Sigma, St. Louis, MO, USA) was used. Immediately prior to its use, a 3% H2O2 solution was added, used for staining for 5 minutes, washed with 0.05 M TBS solution and distilled water, and counterstained for one minute using Nuclear Fast Red, before it was then washed with distilled water and mounted with permount after the clarifying process.
All of the stained samples were examined under an Olympus AX-70 light microscope, and pictures were taken using a Leica DC200 digital camera. As a result of the immunohistochemical stain, according to the color intensity in the nucleus of the neurons, reactions were evaluated as negative (-), meagerly positive reaction (±), weakly positive (+), moderately positive (++), or strong positive (+++). Quantitative measurements of the color intensity were performed by 2 independent observers (JHL and TKP) in a masked fashion. For statistical analysis, we scored the results of immunohistochemical staining and used the Mann-Whitney U-test. A p-value of less than 0.05 was considered statistically significant.
In the control group (immediately after birth), the dorsal and ventral nuclei in the LGN of albino rats could not be distinguished clearly (Fig. 1A). Small undifferentiated neurons with strong basophilic nuclei were observed. The nuclei showing weak basophilic characteristics were surrounded by an extremely small number of neurons in their early differentiation phase, with thin cytoplasm showing strong basophilic characteristics (Fig. 1B). In the control group (1 week after birth), the dorsal and ventral LGN were easy to distinguish based on the primitive intergenicular leaflet, and the myelinated nerve fibers were not developed. In the dorsal LGN, a small number of large neurons with the distinct nucleoplasm and the basophilic cytoplasm were detected. Most neurons contained a meager basophilic vesicular nucleus, and the development of cytoplasm was insufficient; nonetheless, they were clearly distinguishable from astrocytes (Fig. 1C). In the experimental group (1 week after birth), a small number of large neurons with a distinct nucleolus and basophilic cytoplasm were observed in the dorsal LGN. Most neurons had the vesicular nuclei showing basophilic characteristics, and the development of the cytoplasm was meager; nonetheless, they could be clearly distinguished from astrocytes (Fig. 1D). In the control group (2 weeks after birth), large neurons with a vesicular nucleus and abundant cytoplasm and small neurons with a vesicular nucleus surrounded by thin cytoplasm could be clearly distinguished in the dorsal LGN. Astrocytes with a condensed and dark nucleus were observed (Fig. 1E). In the experimental group (2 weeks after birth), more large neurons with many small vesicular structures in the cytoplasm in the dorsal LGN were observed than in the age-matched control group (Fig. 1F). In the control group (4 weeks after birth), large neurons with a vesicular nucleus and abundant cytoplasm and small neurons with a vesicular nucleus surrounded by thin cytoplasm could be clearly distinguished in the dorsal LGN, suggesting normal neuronal development (Fig. 1G). In the experimental group (4 weeks after birth), the cytoplasm of the large neurons became vacuolized. The basophilic characteristics of the Nissl body could not be detected in the dorsal LGN, suggesting neuronal degeneration (Fig. 1H).
The control group (immediately after birth, 5 neonates) was excluded because there was no comparable age-matched experiment group. In the control group (1 week after birth), c-Fos immunoreactivity was not detected in the nucleus of the neurons in the dorsal LGN (Fig. 2A). In the experimental group (1 week after birth), meager c-Fos immunoreactivity was observed in the nucleus of the neurons in the dorsal LGN (Fig. 2B). In the control group (2 weeks after birth), c-Fos immunoreactivity was not detected in the nucleus of the neurons in the dorsal LGN (Fig. 2C). In the experimental group (2 weeks after birth), granular weak c-Fos immunoreactivity was observed in the nucleus of the neurons in the dorsal LGN (Fig. 2D). In the control group (4 weeks after birth), meager or weak c-Fos immunoreactivity was detected in granular or fibrous patterns in the nucleus of the neurons in the dorsal LGN (Fig. 2E). In the experimental group (4 weeks after birth), moderate or strong c-Fos immunoreactivity was observed in the nucleus of the neurons in the dorsal LGN (Fig. 2F). The color intensity scores in the nucleus of the neurons were 0.00 ± 0.000 in the control group and 1.00 ± 0.667 in the experimental group at 1 week after birth (p = 0.013); 1.20 ± 0.447 in the control group and 2.10 ± 0.876 in the experimental group at 2 weeks after birth (p = 0.04); and 2.20 ± 0.447 in the control group and 3.20 ± 0.789 in the experimental group at 4 weeks after birth (p = 0.04) (Table 1).
In mammals, immediately after birth, the LGN and the visual cortex are in the structurally and functionally immature state, and they mature gradually with light stimulation during a certain period after birth, known as the critical period. Generally, the critical period of the visual system in albino rats is from the middle of the second week to about 45 days after birth [9]. Particularly during the critical period, if appropriate visual stimulation is deprived, the visual system will remain in an immature state [10-12].
Together with c-jun, egr-1, and others, attention has been paid to c-Fos as a neurobiological marker of the brain activity related to light stimulation [13]. The c-Fos gene is a type of immediate early proto-oncogene that reacts to mitogenic stimuli, and it has been reported to be involved in diverse cellular processes such as proliferation, differentiation, transformation, and apoptosis. Mammalian c-Fos is a 4 Kb gene with 4 exons, and it contains a 2.2 kb mRNA transcription portion and synthesizes the c-Fos protein composed of 381 amino acids [14]. The Fos family of proteins contains hydrophobic bzip that mediates the protein-protein interactions. The Fos family of proteins forms heterodimers with the diverse Jun family of proteins, thus forming activator protein-1 (AP-1). AP-1 has been reported to play an important role in cellular proliferation, and it was the first discovered mammalian sequence-specific transcription factor. In unstimulated cells, the c-Fos protein is maintained at the basal level, and synthesis is induced temporarily in response to not only biological stimulations such as neurotransmitters and growth factors, but also environmental changes such as light stimulation. In addition, Shaulian and Karin [15] have reported that c-Fos was associated with biological phenomena such as cell cycle progression and tumorigenesis and with increased activity of c-Fos in the skeletal system, the central nervous system, and the hematopoietic system including megakaryocytes during development has been revealed [16-19]. In neurons, the synthesis of c-Fos is induced by neurotransmitters or light stimulation. The axons originating from the neurons in the dorsal LGN are primarily projected to the visual cortex, whereas the axons from neurons in the ventral LGN have been known to be mostly projected to the pretectal area of the midbrain, the superior colliculus, and the structures controlling the reflection related to the visual sense such as the suprachiasmatic nucleus of the hypothalamus.
In the visual cortices of mammals, visual stimulation during the critical period and age-dependent development play important roles in the overall development. Deprivation of visual stimulation due to light deprivation by rearing in the dark or with eyelid sutures impairs the normal development and maturation of various structures in the visual system [12]. This period when the plasticity of neurons is very high is termed the critical period. In albino rats, the critical period of the visual cortex begins at 1 to 15 days after birth, when neonatal albino rats open their eyes, and continues up to 45 days after birth [9]. Mower and Kaplan [8] have reported that the critical period in cats begins approximately 3 weeks after birth, reaching the peak 4 to 5 weeks after birth, subsequently decreasing at 20 weeks, and terminating approximately 1 year after birth [20]. Fosse et al. [13] have reported in a study conducted on cats maintained in a dark environment that appropriate visual stimulation played a more important role in the development and maturation process of the visual cortex during the critical period than does simple age-dependent maturation. Mower and Kaplan [8] have reported that the critical period was prolonged in comparison with that in cats who received normal visual stimulation when cats were raised in a dark room prior to the critical period, whereas the plasticity of the visual cortex was higher in 20-week-old cats reared in a dark environment than it was in the normal controls.
The expression of IEGs is involved in the development and maturation process of the visual system. Ohki et al. [21] have reported that on the 5th day, the 10th day, the 15th day, and the 100th day after birth, albino rats were raised in an environment of alternating 12 hour-unit light/dark cycles and exposed to light for 30 minutes prior to sacrifice. They were sacrificed at 8:30 AM when the c-Fos gene reached the peak level in the inner nuclear layer of the retina or at 2:30 PM when it reached the peak level in the outer nuclear layer of the retina. After examining the expression of the c-Fos gene, it was observed that the expression of c-Fos could not be detected in the retinas of 5-day-old or10-day-old neonatal albino rats; although it was observed in 15-day-old rats. Thus, in neonatal albino rats, the time when the expression of c-Fos began in response to the light exposure is between 11 days and 15 days after birth [21].
In this study, overall c-Fos immunoreactivity in the experiment groups was significantly increased in the dorsal LGN in comparison with that in the age-matched control group. Such results are thought to be attributed to the fact that the albino rats in the control groups received sufficient continuous stimulation by sunlight, thus the normal basal level of c-Fos immunoreactivity was detected. The abnormal morphology of the visual relay neurons suggesting neuronal degeneration was observed in the dorsal LGN in the experimental group. Therefore, if visual stimulations are deprived prior to the critical period, the impairment of the morphological and functional maturation of the LGN will be induced in albino rats, and it is thought that the c-Fos gene plays an important role in the morphological and functional development and maturation in the LGN, similar to other structures of the visual system, such as the retina, the superior colliculus, and the visual cortex.
Figures and Tables
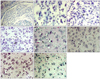 | Fig. 1The morphological assessment. (A) Control group (immediately after birth). Lateral geniculate body is composed of small undifferentiated neurons which form short cellular cords scattered within the nucleus. The dorsal and ventral lateral geniculate nucleus are not yet well distinguished. Few myelinated fibers are seen. Luxol fast blue as well as cresyl violet (LFB-CV), ×100. (B) Control group (immediately after birth). Most cells with darkly stained nuclei are undifferentiated. LFB-CV, ×400. (C) Control group (1 week after birth). Neurons (arrow) with a vesicular nucleus are well distinguished from astrocytes (white arrow), in which the condensed nucleus is composed of darkly stained chromatin. The cytoplasm of the neurons is still poorly developed so that it appears as a thin rim surrounding the nucleus. LFB-CV, ×400. (D) Experimental group (1 week after birth). Neurons (arrow) with a vesicular nucleus are well distinguished from astrocytes (white arrow), in which the condensed nucleus is composed of darkly stained chromatin. The cytoplasm of the neurons is still poorly developed so that it appears as a thin rim surrounding the nucleus. LFB-CV, ×400. (E) Experimental group (1 week after birth). Large neurons with a vesicular nucleus and vacuolated cytoplasm (arrow) are observed. Astrocytes with a condensed and dark nucleus (white arrow) are seen. Hematoxylin and eosin (H&E), ×400. (F) Experimental group (2 weeks after birth). Large neurons with vacuolated or vesicular cytoplasm are frequently seen. H&E, ×400. (G) Control group (4 weeks after birth). Neurons with well developed basophilic cytoplasm are observed. Astrocyte and oligodendrocyte are also seen. H&E, ×400. (H) Experimental group (4 weeks after birth). Most of the large neurons have vesicular cytoplasm. Small neurons (arrow) with thin basophilic cytoplasm are spared. H&E, ×400. |
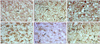 | Fig. 2The immunohistochemical assessment. (A) Control group (1 week after birth). No c-Fos immunoreactivity was observed in the nucleus (arrow). ABC method, ×400. (B) Experimental group (1 week after birth). The nucleus of the neurons shows trace c-Fos immunoreactivity (arrow). ABC method, ×400. (C) Control group (2 weeks after birth). No c-Fos immunoreactivity was observed in the nucleus (arrow). ABC method, ×400. (D) Experimental group (2 weeks after birth). Weak granular c-Fos immunoreactivity is observed in the nucleus. ABC method, ×400. (E) Control group (4 weeks after birth). Granular trace to weak c-Fos immunoreactivity in the nucleus. ABC method, ×400. (F) Control group (4 weeks after birth). Moderate to strong c-Fos immunoreactivity is observed in the nucleus of the neurons (arrow). ABC method, ×400. |
References
1. Morgan JI, Curran T. Stimulus-transcription coupling in the nervous system: involvement of the inducible proto-oncogenes fos and jun. Annu Rev Neurosci. 1991. 14:421–451.
2. Greenberg ME, Greene LA, Ziff EB. Nerve growth factor and epidermal growth factor induce rapid transient changes in proto-oncogene transcription in PC12 cells. J Biol Chem. 1985. 260:14101–14110.
3. Hamamura M, Ozawa H, Kimuro Y, et al. Differential decreases in c-fos and aldolase C mRNA expression in the rat cerebellum after repeated administration of methamphetamine. Brain Res Mol Brain Res. 1999. 64:119–131.
4. Imaki T, Shibasaki T, Hotta M, Demura H. Intracerebroventricular administration of corticotropin-releasing factor induces c-fos mRNA expression in brain regions related to stress responses: comparison with pattern of c-fos mRNA induction after stress. Brain Res. 1993. 616:114–125.
5. Chaudhuri A, Zangenehpour S, Rahbar-Dehgan F, Ye F. Molecular maps of neural activity and quiescence. Acta Neurobiol Exp (Wars). 2000. 60:403–410.
6. Yoshida K, Kawamura K, Imaki J. Differential expression of c-fos mRNA in rat retinal cells: regulation by light/dark cycle. Neuron. 1993. 10:1049–1054.
7. Lu B, Coffey P, Lund R. Increased c-fos-like immunoreactivity in the superior colliculus and lateral geniculate nucleus of the rd mouse. Brain Res. 2004. 1025:220–225.
8. Mower GD, Kaplan IV. Fos expression during the critical period in visual cortex: differences between normal and dark reared cats. Brain Res Mol Brain Res. 1999. 64:264–269.
9. Wang WF, Kiyosawa M, Ishiwata K, Mochizuki M. Glucose metabolism in the visual structures of rat monocularly deprived by eyelid suture after postnatal eye opening. Jpn J Ophthalmol. 2005. 49:6–11.
10. Cynader M, Mitchell DE. Prolonged sensitivity to monocular deprivation in dark-reared cats. J Neurophysiol. 1980. 43:1026–1040.
11. Mower GD, Kaplan IV. Immediate early gene expression in the visual cortex of normal and dark reared cats: differences between fos and egr-1. Brain Res Mol Brain Res. 2002. 105:157–160.
12. Rosen KM, McCormack MA, Villa-Komaroff L, Mower GD. Brief visual experience induces immediate early gene expression in the cat visual cortex. Proc Natl Acad Sci U S A. 1992. 89:5437–5441.
13. Fosse VM, Heggelund P, Fonnum F. Postnatal development of glutamatergic, GABAergic, and cholinergic neurotransmitter phenotypes in the visual cortex, lateral geniculate nucleus, pulvinar, and superior colliculus in cats. J Neurosci. 1989. 9:426–435.
14. Piechaczyk M, Blanchard JM. c-Fos proto-oncogene regulation and function. Crit Rev Oncol Hematol. 1994. 17:93–131.
15. Shaulian E, Karin M. AP-1 in cell proliferation and survival. Oncogene. 2001. 20:2390–2400.
16. Alitalo R, Partanen J, Pertovaara L, et al. Increased erythroid potentiating activity/tissue inhibitor of metalloproteinases and jun/fos transcription factor complex characterize tumor promoter-induced megakaryoblastic differentiation of K562 leukemia cells. Blood. 1990. 75:1974–1982.
17. Caubet JF, Mitjavila MT, Dubart A, et al. Expression of the c-fos protooncogene by human and murine erythroblasts. Blood. 1989. 74:947–951.
18. Dony C, Gruss P. Proto-oncogene c-fos expression in growth regions of fetal bone and mesodermal web tissue. Nature. 1987. 328:711–714.
19. Smeyne RJ, Curran T, Morgan JI. Temporal and spatial expression of a fos-lacZ transgene in the developing nervous system. Brain Res Mol Brain Res. 1992. 16:158–162.
20. Olson CR, Freeman RD. Profile of the sensitive period for monocular deprivation in kittens. Exp Brain Res. 1980. 39:17–21.
21. Ohki K, Yoshida K, Harada T, et al. c-fos gene expression in postnatal rat retinas with light/dark cycle. Vision Res. 1996. 36:1883–1886.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download