Abstract
Purpose
To describe the ophthalmoscopic features and natural history in a case series of eyes that developed intraocular hemorrhages associated with perinatal distress and to evaluate their clinical courses.
Methods
A retrospective chart review of 289 neonates with a medical history of perinatal distress was conducted. Among these 289 patients (578 eyes), 29 eyes of 17 neonates were found to have had retinal hemorrhages or vitreous hemorrhages (VH). A comprehensive chart review, including details of fundoscopic findings and perinatal history, was conducted.
Results
Intraocular hemorrhage was present in 5.5% of the patients. Most hemorrhages (82.7%) were intraretinal. In our population, 17% (n = 5) of hemorrhages resolved within two weeks, but 31% (n = 9) did not resolve even after four weeks. Most hemorrhages spontaneously resolved without any specific sequelae; however, one infant's dense unilateral VH persisted up to three months after birth. When the patient was seen again at 3.5 years of age, she had developed axial myopia and severe amblyopia of the involved eye.
Conclusions
In asphyxiated newborns, the possibility of intraocular hemorrhages should be considered. Long-standing, dense hemorrhages obscuring the macula may lead to severe vision deprivation amblyopia. Therefore, ophthalmic examination should be considered in neonates with perinatal distress, and close observation is necessary for hemorrhages that do not resolve in this amblyogenic age group.
There are several potential causes of retinal hemorrhages (RH) in neonates. Hemorrhages at birth may occur after traumatic deliveries; neonatal coagulopathies are associated with sepsis, shaken baby syndrome and intracranial hemorrhage [1]. The reported incidence of neonatal retinal hemorrhages varies widely, from 2.6% to 50%, which is possibly due to different patient demographics, how soon after birth examinations are conducted, and different examination techniques [2]. In general, birth-related RH resolves quickly and does not cause subsequent visual or neurological deficits [2]. Despite the number of articles documenting the incidence and duration of RH in healthy newborns, there have been no prior reports on RH in asphyxiated newborns.
Asphyxia or respiratory distress is defined as a condition of impaired gas exchange that leads to hypoxemia, hypercapnia and metabolic acidosis [3]. Clinical criteria include the following: abnormalities in electronic fetal monitoring, meconium-stained amniotic fluid, metabolic acidemia, low Apgar scores, and post-asphyxia neurological and/or extra neurological abnormalities [4].
In this report, we discuss the incidence, pattern and duration of RH and vitreous hemorrhages (VH) associated with perinatal distress in newborns and evaluate their clinical significance.
We retrospectively reviewed medical records of perinatally distressed newborns hospitalized at the Neonatal Intensive Care Unit of our hospital between March 2006 and December 2009 who were referred for ophthalmic examination by the pediatrics department. Perinatal distress included the following: birth asphyxia, meconium aspiration, amniotic fluid aspiration, fetal distress, transient tachypnea of the newborn, and dysphagic choking. The Institutional Review Board at our hospital approved this retrospective chart review. We identified 289 patients. Among these patients, 17 were noted to have RH or VH. Our department performed a detailed chart review, including comprehensive ocular findings and perinatal history. All RH or VH cases were diagnosed by indirect ophthalmoscopy and scleral depression with proparacaine 0.5% topical anesthetic. The shape, location and other associated features of the hemorrhages were noted for each eye in a drawing of the fundus. Hemorrhages were classified according to their location in three retinal zones [2,5].
The newborns were reexamined every one or two weeks until the hemorrhages resolved completely. In one participant with unilateral VH, the follow-up continued until the child was 3.5 years old.
The mean age at the first funduscopic examination was 11.5 days. Among the 17 infants, 11 (65%) had bilateral hemorrhages. The RH were dot, blot or flame shaped (Fig. 1). Among the 29 eyes with hemorrhages, 82.7% (n = 24) were intraretinal; one neonate had unilateral preretinal hemorrhages, and another had bilateral subretinal hemorrhages. Two neonates had unilateral VH. Table 1 summarizes the demographic information for those infants found to have RH or VH. The perinatal histories, pattern, distribution, and duration of the hemorrhages are shown in Table 2.
The newborns were reexamined every one to two weeks until the hemorrhages were completely resolved. The average duration of hemorrhages after the first examination was 5.1 weeks. Among the 29 eyes, only 17% (n = 5) of hemorrhages resolved within two weeks; 31% (n = 9) did not resolve completely even after four weeks. The one hemorrhage that persisted for up to three months was a case with dense unilateral VH. The posterior pole of the involved eye was almost completely obscured. B-scan ultrasonography showed a diffuse VH concentrated at the macular area (Fig. 2A). Both retinas were otherwise normal with no sign of infection or other abnormality. Even after complete resolution, the cause of the VH could not be determined. When the patient was seen again at 3.5 years of age, she had developed severe amblyopia. A cycloplegic refraction revealed +1.5 D sph in the right eye and -3.50 D sph = -1.5 D cyl × 180° A in the left eye.
RH can occur in healthy newborn infants during delivery. The reported incidence varies from 2.6 to 50% [2]. Giles [6] found that the incidence was reduced from 40% at 1 hour post delivery to 20% at 72 hours. Sezen [7] showed the incidence was only 2.6% after three to five days. A study by Kim et al. [8] reported the prevalence of neonatal RH in Korea to be 19.1% at 24 hours after birth. The presence of RH in healthy newborns presumably relates to the birth process, and spontaneous absorption generally occurs over time.
Among the 289 perinatally distressed newborns in the current retrospective chart review, 17 neonates were found to have RH or VH. The proportions of cases having hemorrhages in this study (5.5%) were lower than values given in several studies of healthy newborns mentioned previously. However, the mean age at first ophthalmic examination in the current study was 11.5 days after birth. Therefore, a direct comparison of the incidence of RH or VH between healthy infants and distressed newborns may not be valid here.
In general, two consensuses exist in the literature regarding RH in healthy newborns. First, the most important factor associated with a greater risk of hemorrhage was instrument- (vacuum or forceps) assisted delivery. Because deliveries assisted with instruments are currently on the decrease in Korea, the actual incidence of birth-related RH is expected to be much lower than the reported values of the past. There were no cases of instrument-assisted deliveries in this study; nevertheless, it is noteworthy that the incidence of RH or VH was 5.5% in the current study even after more than one week of age.
Second, hemorrhages of healthy newborns resolve quickly, and most do not cause subsequent visual deficits. Emerson et al. [2] and Hughes et al. [9] reported that approximately 90% of RH detected at birth resolved within two weeks, and none were detectable four weeks after birth. Therefore, very few RH could have been found in healthy infants at our first examination because of the participants' age. In additions, the average duration of hemorrhages (5.1 weeks) after first examination in our case series of asphyxiated newborns was much longer than that of healthy babies.
The history of perinatal distress in this study included the following: birth asphyxia, meconium aspiration, placental insufficiency, transient tachypnea, pneumonia, and dysphagic choking. Under these conditions, intraocular hemorrhages might result from significant hypoxia [10]. Geddes et al. [11] suggested that hypoxia leading to intracranial problems might be a cause of RH. Autoregulatory hypoxic cerebral vasodilatation produces an increase in intracranial pressure, which in turn increases the retinal venous pressure [11,12]. Moreover, sustained or episodic systemic arterial hypertension and hypoxic related vascular fragility increases the risk for intraocular hemorrhages [11]. These are also the proposed mechanisms associated with the RH or VH in Terson's syndrome and high altitude retinopathy [13-15]. Venous stasis and congestion are among the proposed causes of RH in Purtscher's retinopathy as well as in cardiopulmonary resuscitation [16].
Similarly, RH or VH in newborns with meconium aspiration may be explained by the same physiological mechanisms. Although there are many factors that trigger the passage of meconium in utero, fetal hypoxia is thought to be the predominant underlying pathophysiology [17,18]. Because meconium passage results from neural stimulation of a mature gastrointestinal tract, meconium aspiration primarily affects term and post-term infants [17]. This reason is the most plausible explanation for why all neonates except one (participant 16) with RH or VH in the current study were full-term babies; the mean gestational age of the infants with hemorrhages was 39.5 weeks.
In addition to the secondary pathophysiological responses to hypoxia, another possible explanation is based on mechanical effects. Aspirated meconium, amniotic fluid, blood, or any source of airway irritation can cause mechanical obstruction. The forceful effort to extrude the irritant material may increase intra-thoracic pressure and subsequently lead to cephalic venous congestion. RHs or VHs are thought to be caused by the rupture of superficial retinal capillaries as a result of increased venous pressure. This process is a well-described mechanism associated with the RH of valsalva retinopathy [19], and this mechanism is also applicable to the case of bilateral RH with a history of choking while breastfeeding in this study [20].
Among the 17 patients in the current study, one unilateral dense VH in a newborn (participant 17) had hemorrhages that remained for up to three months. Although the pattern and duration of hemorrhages did not appear to be related to the severity of the perinatal distress in the current study, it is of interest to note that this neonate had the most severe asphyxia at birth. The dense VH in the infant resolved almost completely at three months after the onset of the hemorrhage (Fig. 2B). The follow-up examination at 3.5 years of age found a subnormal fixation and following pattern of the involved eye with 3+ left inferior oblique muscle overaction. Fundoscopic examination showed left excyclotropia (Fig. 2C). The cycloplegic refraction was +1.5 D sph in the right eye and - 3.50 D sph = - 1.5 D cyl × 180° A in the left eye. Spirn et al. [21] reported that infants with VH may be more likely to manifest strabismus or nystagmus due to the immaturity of the visual system, and visual deprivation from the vitreous hemorrhage may also result in amblyopia. Furthermore, Mohney [22] described four infants with dense unilateral VH in the first weeks of life (three of four infants underwent vitrectomy between three and six weeks after hemorrhage onset). He reported that the presence of dense vitreous blood led to severe amblyopia and significant myopic shifts over 10 D in the involved eyes. The finding of a myopic shift in this study was also consistent with the known facts that certain forms of visual deprivation of the infantile eye (congenital cataracts, corneal opacities, ptosis, and VH) can lead to axial myopia [22-26].
The exact duration of a dense hemorrhage needed to cause abnormal myopic development and significant amblyopia is unclear. Several reports have recommended waiting from four weeks to several months [27,28]. By contrast, Mohney [22] suggested that early surgical intervention for dense VH of the newborn should be performed based on the findings that all three neonates who underwent vitrectomy between three and six weeks after the onset of the hemorrhage developed severe axial myopia and irreversible amblyopia.
The limitations of this study include the retrospective study design and the small number of patients. Another weakness was the initial examination time of 11.5 days after birth. Several prior studies of healthy newborn infants during delivery examined the newborns right after birth. However, eye examinations could not be performed in our study until the newborns were medically stable. Large prospective studies with early examinations are needed to confirm the findings of our study.
In summary, newborns with a history of perinatal distress were more likely to have RH or VH than healthy infants. Moreover, the duration of intraocular hemorrhages was much longer than that of healthy babies. Hypoxia with brain swelling, cerebral vessel congestion and hypertension, as well as increased intra-thoracic pressure due to mechanical obstruction, were proposed as the combined underlying pathophysiology. Although the findings of RH or VH seem transient and do not appear to be associated with short-term sequelae related to the hemorrhagic retinopathy, myopia and severe amblyopia may still result from persistent dense hemorrhages that obscure the macula. Therefore, ophthalmic examination should be considered in perinatally distressed newborns for early detection of ocular abnormalities. The location, severity and duration of the hemorrhages require close monitoring and possible surgical intervention for patients with unresolved hemorrhages in this amblyogenic age group.
Figures and Tables
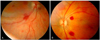 | Fig. 1Fundus photographs of retinal hemorrhages in neonates that had a history of birth asphyxia and amniotic fluid aspiration. (A) Participant 15, (B) participant 9. |
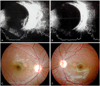 | Fig. 2(A) B-scan ultrasonography of an infant with dense vitreous hemorrhage (participant 16). The infant had a persistent dense vitreous hemorrhage obscuring the macula of the left eye for up to three months. (B) Vitreous hemorrhages were absorbed completely after three months. (C) Fundus photographs of excyclotropia in the left eye at 3.5 years of age. The follow-up examination showed that the patient had developed myopia and severe amblyopia of the involved eye. |
Table 2
Perinatal history and ocular findings of 17 patents

IRH = intraretinal hemorrhage; TTN = transient tachypnea of the newborn; NSVD = normal spontaneous vaginal delivery; VH = vitreous hemorrhage; C-section = cesarean section; RH = retinal hemorrhage; RDS = respiratory distress syndrome; DIC = disseminated intravascular coagulopathy; B = both eyes; R = right eye; L = left eye.
References
1. Shaikh S, Fishman ML, Gaynon M, et al. Diffuse unilateral hemorrhagic retinopathy associated with accidental perinatal strangulation. A clinicopathologic report. Retina. 2001. 21:252–255.
2. Emerson MV, Pieramici DJ, Stoessel KM, et al. Incidence and rate of disappearance of retinal hemorrhage in newborns. Ophthalmology. 2001. 108:36–39.
3. Low JA. Intrapartum fetal asphyxia: definition, diagnosis, and classification. Am J Obstet Gynecol. 1997. 176:957–959.
4. Gonzalez de Dios J. Definition of perinatal asphyxia in medical literature: the need of a consensus. Rev Neurol. 2002. 35:628–634.
5. AIDS Clinical Trials Group (ACTG). Studies of ocular complications of AIDS Foscarnet-Ganciclovir Cytomegalovirus Retinitis Trial: 1. Rationale, design, and methods. Control Clin Trials. 1992. 13:22–39.
6. Giles CL. Retinal hemorrhages in the newborn. Am J Ophthalmol. 1960. 49:1005–1011.
7. Sezen F. Retinal haemorrhages in newborn infants. Br J Ophthalmol. 1971. 55:248–253.
8. Kim KH, Rhee MG, Shin TY. Retinal hemorrhages in newborn infants. J Korean Ophthalmol Soc. 1980. 21:441–444.
9. Hughes LA, May K, Talbot JF, Parsons MA. Incidence, distribution, and duration of birth-related retinal hemorrhages: a prospective study. J AAPOS. 2006. 10:102–106.
10. Madan A, Hamrik S, Ferriero DM. Taeusch HW, Ballard RA, Gleason CA, editors. Central nervous system injury and neuroprotection. Avery's diseases of the newborn. 2005. 8th ed. Philadelphia: Elsevier Saunders;971–983.
11. Geddes JF, Tasker RC, Hackshaw AK, et al. Dural haemorrhage in non-traumatic infant deaths: does it explain the bleeding in 'shaken baby syndrome'? Neuropathol Appl Neurobiol. 2003. 29:14–22.
12. Ladjimi A, Zaouali S, Messaoud R, et al. Valsalva retinopathy induced by labour. Eur J Ophthalmol. 2002. 12:336–338.
13. Smith DC, Kearns TP, Sayre GP. Preretinal and optic nerve-sheath hemorrhage: pathologic and experimental aspects in subarachnoid hemorrhage. Trans Am Acad Ophthalmol Otolaryngol. 1957. 61:201–211.
14. Gardner H. Correlation between retinal abnormalities and intracranial abnormalities in the shaken baby syndrome. Am J Ophthalmol. 2003. 135:745.
15. Shults WT, Swan KC. High altitude retinopathy in mountain climbers. Arch Ophthalmol. 1975. 93:404–408.
16. Goetting MG, Sowa B. Retinal hemorrhage after cardiopulmonary resuscitation in children: an etiologic reevaluation. Pediatrics. 1990. 85:585–588.
17. Van Ierland Y, de Beaufort AJ. Why does meconium cause meconium aspiration syndrome? Current concepts of MAS pathophysiology. Early Hum Dev. 2009. 85:617–620.
18. Tyler DC, Murphy J, Cheney FW. Mechanical and chemical damage to lung tissue caused by meconium aspiration. Pediatrics. 1978. 62:454–459.
19. Duane TD. Valsalva hemorrhagic retinopathy. Trans Am Ophthalmol Soc. 1972. 70:298–313.
20. Barnes PD, Galaznik J, Gardner H, Shuman M. Infant acute life-threatening event: dysphagic choking versus nonaccidental injury. Semin Pediatr Neurol. 2010. 17:7–11.
21. Spirn MJ, Lynn MJ, Hubbard GB 3rd. Vitreous hemorrhage in children. Ophthalmology. 2006. 113:848–852.
22. Mohney BG. Axial myopia associated with dense vitreous hemorrhage of the neonate. J AAPOS. 2002. 6:348–353.
23. Von Noorden GK, Lewis RA. Ocular axial length in unilateral congenital cataracts and blepharoptosis. Invest Ophthalmol Vis Sci. 1987. 28:750–752.
24. Gee SS, Tabbara KF. Increase in ocular axial length in patients with corneal opacification. Ophthalmology. 1988. 95:1276–1278.
25. Hoyt CS, Stone RD, Fromer C, Billson FA. Monocular axial myopia associated with neonatal eyelid closure in human infants. Am J Ophthalmol. 1981. 91:197–200.
26. Miller-Meeks MJ, Bennett SR, Keech RV, Blodi CF. Myopia induced by vitreous hemorrhage. Am J Ophthalmol. 1990. 109:199–203.
27. Ferrone PJ, de Juan E Jr. Vitreous hemorrhage in infants. Arch Ophthalmol. 1994. 112:1185–1189.
28. Isenberg SJ. Isenberg SJ, editor. Acquired disorders of the infant eye, ocular trauma. The eye in infancy. 1994. St. Louis: Mosby;487.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download