Abstract
Purpose
To compare the efficacy between intravitreal bevacizumab and combination treatment (bevacizumab and macular photocoagulation) for the treatment of diabetic macular edema (DME). In addtion, changes of DME type were researched using optical coherence tomography.
Methods
The present study included 90 eyes with bevacizumab injection and 38 eyes with combination treatment. Using chart records, patients were reviewed until 6 months after treatment. The present study compared changes of visual acuity (VA) and macular thickness at each follow up. DME was classified into 4 types and the morphologic pattern was compared.
Results
In patients with the bevacizumab injection only, VA improved from 0.29 ± 0.18 to 0.48 ± 0.26 at 1 month and returned to 0.32 ± 0.20 at 6 months after treatment. In the combination treatment, VA improved from 0.32 ± 0.22 to 0.52 ± 0.26 at 1 month and returned to 0.36 ± 0.18 at 6 months after treatment. There was no significant improvement of VA at the final follow-up with either treatment. There was significant decrease of macular thickness except in the mixed DME type.
Diabetic retinopathy is the leading cause of blindness in people of working age. Diabetic macular edema (DME) affects approximately 29% of diabetic patients with disease duration of 20 years or more and is the main reason for reduced vision in this segment of population [1]. The Early Treatment Diabetic Retinopathy Study (ETDRS) showed the 3-year risk of moderate visual loss for diabetic patients with clinically significant macular edema was 30% [2]. In the ETDRS study, laser photocoagulation reduced the risk of moderate visual acuity loss for all eyes with DME and mild to moderate non-proliferative diabetic retinopathy by approximately 50% [3-5]. However, 12% of the treated eyes still lost 15 or more ETDRS letters at the 3-year follow-up interval [6,7]. Furthermore, less than 3% of treated eyes demonstrated an improvement in visual acuity of the same magnitude [4]. Over time, laser burns may develop into areas of progressive retinal pigment epithelium and retinal atrophy that become larger than the original laser spot size and encroach on fixation. Photocoagulation for DME may be associated with central scotomas associated with loss of central vision and decreased color vision [8,9].
In attempt to reduce these limitations, intravitreal triamcinolone acetonide (TA) or anti-vascular endothelial growth factor (VEGF) agent was injected in patients with DME and favorable results were shown in previous studies [10-13]. However, intravitreal TA or anti-VEGF agent showed temporary effects and repeated treatments were needed [14-16]. Many retina specialists have studied brisk treatment modalities to cure DME with significant results discovered [16]. However, limitations still exist such as refractory, recurrent DME, ideal regimen, appropriate numbers of injections and the time of injection interval.
To overcome the limitation of DME treatment, various therapeutic options were studied. Lam et al. [17] reported comparable results of intravirtreal TA plus sequential grid laser versus TA or laser alone. Their results showed combination treatment did not yield a better effect for treating DME. Kim et al. [18] reported another result regarding a different effect of intravitreal TA according to the DME morphologic type. To the authors' knowledge, to date there are no reported results regarding the combination treatment of intravitreal bevacizumab plus macular laser or different effects of intravitreal bevacizumab injection according to DME pattern and change of DME pattern after the bevacizumab injection.
Therefore, the authors of the present study investigated the efficacy of intravitreal bevacizumab injection alone or in combination with macular photocoagulation and the change of DME pattern with optical coherence tomography (OCT) findings after each treatment.
This retrospective study was approved by the Review Board/Ethics Committee of our hospital.
The present study included 128 eyes of 120 patients from May 2008 to May 2009. Patients were treated with intravitreal bevacizumab injection or intravitreal bevacizumab injection plus macular laser for DME. The macular edema was diagnosed on slit lamp biomicroscopy and confirmed with either fluorescein angiography (FAG) or OCT. Exclusion criteria included macular edema secondary to causes other than diabetic retinopathy, signs of vitreomacular traction, aphakia and history of glaucoma or ocular hypertension. Patients who had panretinal photocoagulation within 6 months, macular laser or intravitreal TA / bevacizumab injection within 12 months, or significant media opacities were also excluded.
Through retrospective chart review, patients were grouped into the bevacizumab injection only group or bevacizumab plus macular laser combination group. Bevacizumab (Avastin; Genentech Inc., South San Francisco, CA, USA) was injected into the vitreous at a dose of 2.5 mg in 0.1 mL. In case of combination treatment, focal or grid macular laser was used within approximately 1 month after bevacizumab injection according to investigator's decision following FAG findings. Every patient underwent complete ophthalmic examination including best-corrected visual acuity, slit lamp examination, intraocular pressure measurement and funduscopic examination after pupil dilation. Reinjection was performed when macular edema still remained or recurred (50 µm or more increased in OCT) or visual acuity was decreased during the follow-up period.
Visual acuity, intraocular pressure, slit lamp and funduscopic examination results were analyzed with chart review at the time of pre-treatment and post-treatment after 2 weeks, 1 month, 2 months, 3 months and 6 months. Morphologic pattern of DME and central macular thickness and volume were also analyzed comparing pre-treatment OCT and post-treatment OCT.
Among 128 eyes, eyes were excluded with mechanical traction on macula or when interpretation was not possible and the remaining 102 eyes (79.7%) with pre- and post-injection OCT results were analyzed by DME pattern. The pattern of DME was classified according to Otani et al. [19], such as diffuse macular edema, cystoids macular edema and serous retinal detachment. Unlike other studies, mixed macular edema, a combination of the above 2 or more types of DME, was used in the present study (Fig. 1).
The data were presented as the mean ± standard deviation. Statistical differences between pre- and post-treatment clinical data were assessed using a paired t-test. Differences between bevacizumab injection and combination therapy were assessed using an independent t-test (SPSS ver 12.0; SPSS Inc., Chicago, IL, USA). A p-value of less than 0.05 was considered to be statistically significant.
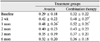
The bevacizumab injection only group and bevacizumab plus macular laser combination group included 90 eyes from 85 patients and 38 eyes from 35 patients, respectively. Baseline characteristics such as age, sex and intraocular pressure were not statistically significant, however, the number of subjects was significantly smaller (p < 0.05) in the combination treatment group than in the bevacizumab injection only group (Table 1). In the intravitreal bevacizumab injection only gorup, the type of DME before treatment was 44.4% diffuse macular edema, 31.1% cystoid macular edema, 13.3% serous retinal detachment and 11.1% mixed macular edema. In the combination treatment group, the type of DME before treatment was 47.3% diffuse macular edema, 28.9% cystoid macular edema, 9.7% serous retinal detachment and 9.7% mixed macular edema. There was no statistically significant difference of DME type (p = 0.49) between the 2 groups (Table 2). Baseline visual acuity before treatment was 0.29 ± 0.18 in the bevacizumab injection only group and 0.32 ± 0.22 in the combination treatment group and there was no significant differences (p = 0.68) between the 2 groups. Until the last follow-up, mean number of injections was 1.7 times in the bevacizumab injection only group and 1.3 times in the combination treatment group (p = 0.57) and there were no adverse events such as endophthalmitis or significant rise of intraocular pressure and cataractous change.
Change of visual acuity after treatment was 0.42 ± 0.22 and 0.48 ± 0.26 at 2 weeks and 1 month after intravitreal bevacizumab injection only, respectively. In the combination treatment, visual acuity after treatment was 0.48 ± 0.27 and 0.52 ± 0.26 at 2 weeks and 1 month after treatment, respectively. Visual acuity was improved in both groups and when comparing the visual acuity at 2 weeks after treatment, change of visual acuity improvement was faster in the combination treatment group than in the bevacizumab injection only group. However, visual acuity slightly regressed, 0.32 ± 0.20 in the bevacizumab injection only group and 0.36 ± 0.18 in the combination treatment until the last follow-up (Table 3 and Fig. 2).
In bevacizumab injection group, macular thickness before treatment was 468.1 ± 105.0 µm and decreased to 374.4 ± 73.5 µm (p < 0.05) at 1 month after treatment. The mean macular volume was also decreased from 0.74 ± 0.19 µm3 to 0.55 ± 0.11 µm3 (p < 0.05) at 1 month after treatment. In combination treatment group, mean macular thickness was decreased from 457.2 ± 95.2 µm to 349.0 ± 126.0 µm (p < 0.05) and mean macular volume was decreased from 0.71 ± 0.19 µm3 to 0.49 ± 0.14 µm3 (p < 0.05) at 1 month after treatment. These show significant decrease after treatment in both groups (Table 4).
Regarding the type of DME, the macular thickness and volume after treatment significantly decreased in patients with diffuse macular edema, cystoid macular edema and serous retinal detachment in both groups (Table 4). However, in the mixed macular edema group, macular thickness and volume showed no improvement or even deterioration; in the bevacizumab injection only group, macular thickness changed from 523.6 ± 162.0 µm to 532.9 ± 103.0 µm and macular volume changed from 0.90 ± 0.20 µm3 to 0.93 ± 0.12 µm3 after treatment. In the combination treatment group, macular thickness changed from 518.2 ± 132.0 µm to 522.8 ± 87.9 µm and macular volume changed from 0.83 ± 0.18 µm3 to 0.85 ± 0.11 µm3 (p = 0.69).
In the bevacizumab injection only group, 3 out of 29 eyes changed the type of DME after treatment from diffuse macular edema to a combination of diffuse macular edema and serous retinal detachment and another 4 eyes changed to a combination of cystoid and diffuse macular edema after treatment. Eight out of 20 eyes changed from cystoid macular edema to combination of diffuse and cystoid macular edema and another 2 eyes changed to a combination of cystoid macular edema and serous retinal detachment after treatment. Two out of 9 eyes changed from serous retinal detachment to diffuse macular edema and another 2 eyes changed to a combination of serous retinal detachment and diffuse macular edema after treatment. Conversely, in mixed macular edema, 6 out of 7 eyes showed no change of DME type after treatment.
In the combination treatment group, 2 out of 15 diffuse macular edemas changed to a combination of diffuse macular edema and serous retinal detachment and another 3 eyes changed to a combination of cystoid and diffuse macular edema after treatment. Four out of 9 cystoid macular edemas changed to cystoid and diffuse macular edema and another 1 eye changed to cystoid macular edema and serous retinal detachment after treatment. One out of 3 serous retinal detachments changed to diffuse macular edema after treatment. Conversely, 3 out of 3 mixed macular edemas showed no change of DME type even after treatment (Table 5).
Previous studies reported results of a combination treatment of intravitreal TA injection and macular laser photocoagulation or combination of intravitreal TA and bevacizumab injection as a treatment option for DME. However, outcomes of combination therapy showed no further beneficial effects than conventional treatment [17,20,21]. Kim et al. [18] also evaluated the efficacy of intravitreal TA injection according to DME type and concluded the cystoid type of macular edema responded better than other types of macular edema. To the authors' knowledge, visual outcomes of intravitreal bevacizumab injection only and macular laser photocoagulation combination treatment or treatment efficacies according to DME type or change of DME type after treatment have not yet been reported.
The present study showed both intravitreal bevacizumab injection only and combination treatment achieved visual improvement at 1 month after treatment and visual acuity regressed at 3 months after treatment. Although there were no significant differences between the 2 groups, visual acuity in the combination treatment group was better than a single treatment at the last follow-up. As we shown in Fig. 2, 3 months to 6 months showed a decrease of visual acuity in the bevacizumab injection only group, otherwise, the combination treatment group showed relatively no change of visual acuity. The results indicate visual acuity could be maintained in the combination treatment otherwise visual acuity would decrease with the bevacizumab injection only in a longer follow-up period. The results can be explained in accordance with a previous study Bak et al. [22] reported; in DME, macula was diffusely thickened and fluid was accumulated in the outer retinal layer because of the blood-retinal barrier breakdown, therefore, the laser could not deliver sufficient power to the RPE layer. With a TA injection, edema could decrease and retinal penetration could increase, allowing the laser to more easily deliver power.
The present study also assumes macular laser photocoagulation after decreasing macular edema with bevacizumab injection can reduce the recurrence of DME and maintain the visual acuity. However, additional studies with a longer follow-up period are necessary to prove this hypothesis.
The pathogenesis of DME is not yet fully understood. The possible mechanism is increased vascular permeability due to injured capillary endothelium consisting of the blood-retinal barrier [23]. The important factor is the retinal ischemic change produced by vascular microstructure change resulting from high blood sugar [23,24]. The depletion of oxygen causes cytotoxic edema which is the expansion of Muller cells and also causes vasogenic edema by secreting VEGF, prostaglandin and inflammatory cytokines (for example interleukin-6) [25,26]. If the cellular edema and ischemia persist, the outer retinal layer cyst will be made by liquefaction necrosis of Muller cells and other neural tissue [27,28]. Based upon the above pathogenesis, a theory regarding physiologic change of DME deterioration upon time sequence was hypothesized. DME which is diffusely thickened macula resulting from cellular expansion caused by cytotoxic and vasogenic edema would be the first step. As the next step, sponge-like configuration of DME would be made because of microcellular liquefaction necrosis when edema and ischemia persist. If the sponge-like cysts are joined, a cystoid type of DME will develop while serous retinal detachment could be made by joining in a different manner. If the DME becomes worse, mixed macular edema consisting of a detached macular area combining a diffuse or cystoid type of edema will develop.
The present study showed macular thickness and volume was significantly decreased 1 month after treatment in both groups. However, when considering morphologic change of DME type, macular edema was decreased in the diffuse, cystoid and serous retinal detachment type but otherwise, showed no change or even an increase in mixed macular edema in both groups. According to the above hypothesis, the results indicate mixed macular edema, which is the last step in DME, could be considered refractory to DME. The reason could be due to the persistent deterioration of cytotoxic and vasogenic edema causing irreversible cellular necrosis. In both treatment groups, a change of macular edema type after treatment was found; cystoid or serous detachment portion decreased and changed to a diffuse type of edema after both treatments. The result may be due to the bevacizumab injection reducing the retinal capillary vascular permeability. However, mixed macular edema did not respond to treatment because no change of macular edema type was observed even after treatment. The above hypothesis is can be explained by observation of the change in the type of macular edema; starting with DME and changing to cystoid or serous retinal detachment and returning back to DME after treatment.
The present study has several limitations such as a nonrandomized retrospective study and including heterogeneous subjects. The follow-up period was relatively short to prove the hypothesis. The small number of patients in the combination treatment group compared to the bevacizumab injection only treatment group could be another limitation.
In conclusion, there were no significant differences between the bevacizumab injection only treatment group and the bevacizumab injection plus macular photocoagulation combination treatment group through 6 months of follow-up. However, the present study expects a bevacizumab plus macular photocoagulation combination treatment could maintain visual acuity and reduce the recurrence of macular edema. In both treatment groups, macular thickness and volume decreased after treatment except for mixed macular edema and macular edema which changed their type after treatment. According to the above results, macular edema could be hypothesized to progress from diffuse edema to cystoid or serous detachment and to mixed type edema. The results from the present study will help reduce the recurrence of macular edema and to decide the appropriate time of treatment by analyzing the DME morphologic pattern. However, animal model studies regarding changes of retinal cells in DME are needed in the future and a prospective study with a longer follow up period should confirm the results.
Figures and Tables
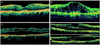 | Fig. 1The classification of diabetic macular edema according to optical coherence tomography features. (A) Diffuse macular edema: thickening of the fovea with homogeneous optical reflectivity throughout the whole layer of the retina. (B) Cystoid macular edema: thickening of the fovea forming cystic space with markedly decreased optical reflectivity in the outer retinal layer. (C) Serous retinal detachment: foveal detachment without vitreomacular traction. (D) Mixed macular edema: combination of the above 3 types of macular edema occupying approximately the same proportion, respectively. |
 | Fig. 2Comparison of visual acuity between intravitreal avastin and intravitreal avastin plus macular laser photocoagulation combination therapy. The asterisk indicates a statistically significant difference within a group (*p < 0.05). |
References
1. Klein R, Klein BE, Moss SE, et al. The Wisconsin epidemiologic study of diabetic retinopathy. IV. Diabetic macular edema. Ophthalmology. 1984. 91:1464–1474.
2. Early Treatment Diabetic Retinopathy Study Research Group. Early photocoagulation for diabetic retinopathy. ETDRS report number 9. Ophthalmology. 1991. 98:5 Suppl. 766–785.
3. Akduman L, Olk RJ. Laser photocoagulation of diabetic macular edema. Ophthalmic Surg Lasers. 1997. 28:387–408.
4. Early Treatment Diabetic Retinopathy Study Research Group. Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Arch Ophthalmol. 1985. 103:1796–1806.
5. Early Treatment Diabetic Retinopathy Study Research Group. Treatment techniques and clinical guidelines for photocoagulation of diabetic macular edema. Early Treatment Diabetic Retinopathy Study Report Number 2. Ophthalmology. 1987. 94:761–774.
6. Wilson DJ, Finkelstein D, Quigley HA, Green WR. Macular grid photocoagulation. An experimental study on the primate retina. Arch Ophthalmol. 1988. 106:100–105.
7. Arnarsson A, Stefansson E. Laser treatment and the mechanism of edema reduction in branch retinal vein occlusion. Invest Ophthalmol Vis Sci. 2000. 41:877–879.
8. Schatz H, Madeira D, McDonald HR, Johnson RN. Progressive enlargement of laser scars following grid laser photocoagulation for diffuse diabetic macular edema. Arch Ophthalmol. 1991. 109:1549–1551.
9. Roider J. Laser treatment of retinal diseases by subthreshold laser effects. Semin Ophthalmol. 1999. 14:19–26.
10. Jonas JB, Kreissig I, Sofker A, Degenring RF. Intravitreal injection of triamcinolone for diffuse diabetic macular edema. Arch Ophthalmol. 2003. 121:57–61.
11. Martidis A, Duker JS, Greenberg PB, et al. Intravitreal triamcinolone for refractory diabetic macular edema. Ophthalmology. 2002. 109:920–927.
12. Haritoglou C, Kook D, Neubauer A, et al. Intravitreal bevacizumab (Avastin) therapy for persistent diffuse diabetic macular edema. Retina. 2006. 26:999–1005.
13. Arevalo JF, Fromow-Guerra J, Quiroz-Mercado H, et al. Primary intravitreal bevacizumab (Avastin) for diabetic macular edema: results from the Pan-American Collaborative Retina Study Group at 6-month follow-up. Ophthalmology. 2007. 114:743–750.
14. Bonini-Filho MA, Jorge R, Barbosa JC, et al. Intravitreal injection versus sub-Tenon's infusion of triamcinolone acetonide for refractory diabetic macular edema: a randomized clinical trial. Invest Ophthalmol Vis Sci. 2005. 46:3845–3849.
15. Jonas JB, Spandau UH, Kamppeter BA, et al. Repeated intravitreal high-dosage injections of triamcinolone acetonide for diffuse diabetic macular edema. Ophthalmology. 2006. 113:800–804.
16. Cunningham ET Jr, Adamis AP, Altaweel M, et al. A phase II randomized double-masked trial of pegaptanib, an anti-vascular endothelial growth factor aptamer, for diabetic macular edema. Ophthalmology. 2005. 112:1747–1757.
17. Lam DS, Chan CK, Mohamed S, et al. Intravitreal triamcinolone plus sequential grid laser versus triamcinolone or laser alone for treating diabetic macular edema: six-month outcomes. Ophthalmology. 2007. 114:2162–2167.
18. Kim YG, Yu SY, Kwak HW. The effect of intravitreal triamcinolone acetonide injection according to the diabetic macular edema type. J Korean Ophthalmol Soc. 2005. 46:84–89.
19. Otani T, Kishi S, Maruyama Y. Patterns of diabetic macular edema with optical coherence tomography. Am J Ophthalmol. 1999. 127:688–693.
20. Avitabile T, Longo A, Reibaldi A. Intravitreal triamcinolone compared with macular laser grid photocoagulation for the treatment of cystoid macular edema. Am J Ophthalmol. 2005. 140:695–702.
21. Ahmadieh H, Ramezani A, Shoeibi N, et al. Intravitreal bevacizumab with or without triamcinolone for refractory diabetic macular edema; a placebo-controlled, randomized clinical trial. Graefes Arch Clin Exp Ophthalmol. 2008. 246:483–489.
22. Bak JH, Chung SE, Kang SW. The short-term effect of triple therapy for diabetic macular edema with vitreomacular traction. J Korean Ophthalmol Soc. 2008. 49:1941–1947.
23. Ferris FL 3rd, Patz A. Macular edema. A complication of diabetic retinopathy. Surv Ophthalmol. 1984. 28:Suppl. 452–461.
24. Antcliff RJ, Marshall J. The pathogenesis of edema in diabetic maculopathy. Semin Ophthalmol. 1999. 14:223–232.
25. Cunha-Vaz J. Diabetic macular edema. Eur J Ophthalmol. 1998. 8:127–130.
26. Lobo C, Bernardes R, Faria de Abreu JR, Cunha-Vaz JG. Novel imaging techniques for diabetic macular edema. Doc Ophthalmol. 1999. 97:341–347.
27. Yanoff M, Fine BS, Brucker AJ, Eagle RC Jr. Pathology of human cystoid macular edema. Surv Ophthalmol. 1984. 28:Suppl. 505–511.
28. Fine BS, Brucker AJ. Macular edema and cystoid macular edema. Am J Ophthalmol. 1981. 92:466–481.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download