Abstract
Purpose
To determine the correlation between the prognosis of branch retinal artery obstruction (BRAO) and the foveal thickness or outer nuclear layer (ONL) thickness on optical coherence tomography (OCT).
Methods
Twenty-one eyes (21 patients) in patients with resolved, non-complicated BRAO and a normal control of 10 eyes (10 volunteers) were used in this study. The average macular thickness, foveal thickness and ONL thickness at central fovea were measured in both the patients and the control group using spectral domain OCT. The thickness between the patient group and the control group were compared and correlation between the best corrected visual acuity (BCVA) and each thickness was determined.
Results
The average age of the patients was 52 ± 5.8 years. The average macular thickness, foveal thickness and ONL thickness at the central fovea of the patients were significantly (p < 0.001, p = 0.023, p = 0.021, respectively) thinner than that of the control group. Both the foveal thickness (rs = 0.56, p = 0.008) and ONL thickness (rs = 0.86, p < 0.001) were significantly correlated with BCVA. There was no significant correlation between the average macular thickness and BCVA.
Branch retinal artery obstructions (BRAO) are thought to represent 38% of all acute retinal artery obstructions [1]. These occlusions usually result from emboli, although nonembolic causes such as vacuities, coagulopathies and vasospasm accompanied by migraines and inflammatory conditions do occur. Emboli are visible 62% of the time upon fundus examination. Risk factors predisposing patients to the development of BRAO include hypertension, carotid artery disease and hypercholesterolemia. BRAO typically occurs at vessel bifurcations with temporal vessels being involved 98% of the time [2].
Compared with central retinal artery obstruction (CRAO), BRAO has more favorable prognoses. The management of BRAO is not aggressive because the visual outcome has been reported to be 20/40 or better in most of the patients affected with BRAO [3-5].
The visual prognosis of BRAO seems to be correlated with presenting visual acuity of the patinet. Eyes with an initial visual acuity of 20/40 or better usually remained at 20/40 or better. Individuals with poor visual acuity such as 20/100 or worse generally did not show any significant improvement [6]. To our knowledge, there is no other report about predictable factors for the prognosis of BRAO except for presenting visual acuity.
Although there had been attempts to find a correlation between the visual prognosis of CRAO and the state of the retina evaluated with optical coherence tomography (OCT) [7], no definite correlation has been reported. Schmidt et al. [7] reported that the thickness of the retina measured by OCT in acute CRAO patients did not have a correlation with visual prognosis and that such an outcome was due to the diversity of retinal swelling in the acute stage. In our study, we examined the state of the retina in chronic BRAO patients through spectral domain OCT (SD-OCT) excluding acute phase in which the retina was diversely swollen. The correlation with the visual prognosis was evaluated by measuring the foveal thickness and outer nuclear layer (ONL) thickness at the fovea.
After the approval of the Institutional Review Board, a retrospective medical record review of 21 consecutive patients (21 eyes) diagnosed with BRAO at Kim's Eye Hospital, Konyang University College of Medicine from January 2008 to May 2009 was performed. Only patients fluorescein angiography exhibited delayed arterial filling and reduced perfusion at the macula in their first visit were included.
Patients having abnormal findings in the anterior segments were excluded. Patients with a history of glaucoma, uveitis and any other ocular disease were also excluded. Patients with concurrent retinal disease, a history of diabetic retinopathy or a history of intraocular surgery were excluded in this study as well. Patients who had a history of systemic hypertension (5 patients) or diabetes (3 patients), were only included if their flurescence angiography showing no other abnormal finding except BRAO. Patients having abnormal optic nerves, severe cataracts, which may have affected visual acuity, or cilioretinal artery obstruction were also excluded from this study. No patient was given treatment for BRAO, but all patients underwent a thorough investigative medical examination, that is not discussed in this article.
Progression of the disease was observed every 2 months after the first medical examination and in the case of patients with the disease where 6 months passed after the onset, the progression was observed every 4 months. Presenting Snellen best-corrected visual acuity (BCVA) obtained at the time of BRAO diagnosis was made and was defined as the baseline BCVA. Follow-up examinations included a funduscopy as well as Snellen BCVA. When no more ischemic change was found in the funduscopy for more than 4 months after the first examination, and when there was no change of the BCVA in the series of observations for more than 2 times, fundus and OCT photograph were taken. A skilled inspector performed OCT by using OTI Spectral OCT/SLO® (Ophthalmic Technologies Inc., Miami, FL, USA).
Raster scan with 32-line of 6 mm was performed for OCT, and the scan depth was 1.5 mm. The thickness of the fovea and average thickness of the macula were measured based on the result from the topographical map analysis. The thickness of the outer nuclear layer (ONL) at the central fovea was measured between the internal limiting membrane (ILM) and the external limiting membrane (ELM) (Fig. 1). Analysis software built in SD-OCT was used for this measurement. Only well-centered scans without overt algorithm failure messages were selected for analysis.
We also performed SD-OCT in 10 control eyes of 10 normal volunteers (5 eyes of 5 men and 5 eyes of 5 women). The average age of the 10 volunteers was 48.5 years (range, 23 to 75 years).
We compared the ONL thickness at the central fovea, foveal thickness and average macular thickness between the subjects and the control group using the Mann-Whitney U-test. We also calculated the Spearman-rank coefficient to study the association between BCVA and each thickness (ONL thickness at the central fovea, foveal thickness and average macular thickness), respectively.
The subjects in this study included 10 males and 11 females with a mean age of 52 ± 5.8 years (range, 25 to 77 years) (Table 1). The average foveal thickness was 145.1 ± 19.3 µm in the patient group and 162.6 ± 21.7 µm in the control group. The average ONL thickness at the central fovea was 112.9 ± 17.5 µm in the patient group and 137.6 ± 19.2 µm in the control group. The average macular thickness was 189 ± 20.5 µm in the patient group and 235.5 ± 20.8 µm in the control group (Table 2).
The foveal thickness (p = 0.023), average ONL thickness (p = 0.021), and average macular thickness (p < 0.001) in the patient group was significantly thinner than the control group (Table 2).
The ONL thickness at the fovea (Spearman-rank correlation coefficient, rs = 0.86; p < 0.001) (Fig. 2) and foveal thickness (rs = 0.56, p = 0.008) (Fig. 2) were positively correlated with BCVA . There was no significant correlation between average macular thickness and BCVA (rs = 0.31, p = 0.186) (Fig. 2).
Branch retinal arteries are mainly distributed in the nerve fiber layer of the retina, and branches of the capillary form the inner capillary and outer capillary plexus. The inner capillary plexus is mainly distributed in the ganglion cell layer while the outer capillary plexus is mainly distributed in the inner nuclear cell layer. Therefore, obstruction of the central retinal artery or branch retinal artery causes ischemic damage in various retinal layers mentioned above.
According to the research that studied the retina of monkeys where the CRAO was derived experimentally, it was reported that the thicknesses of the nerve fiber layer, ganglion cell layer, inner plexiform layer, and inner nuclear layer were decreased when the retina underwent ischemic damage [8]. In the case of BRAO, the retina became swollen to variable degrees immediately after ischemic damage with a gradual decrease of the thickness of the macula over the next 3 months [9].
In this study, the foveal thickness, ONL thickness, and average macular thickness of patients with BRAO decreased compared to the normal control group. This finding was statistically significant (Table 2). These results complied with previous research data of Asefzadeh and Ninyo [10] who reported the changes in OCT findings of BRAO patients.
According to Hayreh et al. [11], when the infarcted retina in BRAO passes through the fovea, the presenting visual acuity is poor, and visual outcome is also poor. However, in our study, there was a case where the presenting visual acuity was not good, and even though the infarcted retina did not pass through the fovea, the vision was finally well recovered (Fig. 1). By suggesting that the retinal arteries perfuse the inner two-thirds of the neural retina and the oxygen of a foveal avascular zone was supplied from choroid [12], it can be assumed that the very center of the fovea is expected to be less influenced by ischemic damage resulting from occlusion of the retinal arteries supplying the inner retina. After all, the survival of the fovea from initial ischemic damage is more important for visual outcome than the extent of the ischemic damage area.
In the current study, the foveal and ONL thickness of the central fovea exhibited a positive correlation with BCVA (Fig. 2). Particularly, in the case of a patient whose presenting vision was not good with a final visual outcome greater than 0.5 (Table 1 and Fig. 1, patient no. 2), the positive final vision could not be explained with existing reports that state presenting vision is important for good visual outcome. It can be predicted that there is a greater possibility of better visual outcomes in cases when more foveal area survives from initial ischemic damage than in cases of patients with less survival of the foveal area. In fact, even in cases when the average macular thickness was greater than others, the visual prognosis is poor when the foveal thickness was thin (Table 1, patient no. 5,12).
Considering ONL thickness that was measured by subtracting outer thickness from the full thickness, it can be easily understood that the correlation between foveal thickness and ONL thickness is similar. However, the correlation coefficient of ONL thickness with BCVA was higher than that of foveal thickness with BCVA (Fig. 2). Since the ONL is comprised of cone cell bodies, decreased ONL thickness suggests the presence of fewer cone cells. When small numbers of cone cells are functional, it is difficult to obtain good vision. In order to recover good visual acuity from ischemic injury, the survival of the ONL is extremely important. Considering the high correlation coefficient with BCVA, our result suggests that ONL thickness may be more important than foveal thickness for good visual outcome. It is possible to presume that good visual outcome is obtained as more photoreceptors are maintained after initial ischemic injury.
The ELM, the junction between the beginning of the inner segment of photoreceptosr and Muller cells, is the outer boundary of the ONL, which is comprised of photoreceptor cell bodies. SD-OCT can detect the ELM with 5 µm axial resolution, which allows for measurement of the ONL thickness. At the central fovea, the inner boundary of the ONL almost abuts the ILM with an intervening layer of Henle fibers. SD-OCT cannot detect this extremely thin Henle fiber layer at the central fovea. Thus, we defined the distance between the ILM and ELM as the ONL thickness at the central fovea. Only this method of measurement of ONL thickness with SD-OCT has been reported so far [13].
According to the previous study that had reported that OCT findings in acute CRAO did not correlate with visual prognosis [7], we assumed that the OCT finding of acute BRAO would not be associated with the prognosis. Therefore, we only included patients in resolved states, except in the acute phase. This decision can be highlighted as the limit of this study. It would be necessary to make a comparative study for foveal thickness in the acute phase and chronic phase.
We failed to demonstrate the relationship between the type of BRAO - transient or permanent - and the visual prognosis for the small number of patients. Ongoing studies will provide a more precise relationship in the future. Although statistical analysis proved to be significant despite the small number of normal controls, an ongoing normative data study is necessary.
In conclusion, foveal thickness and the ONL thickness at the fovea were positively correlated with the BCVA in resolved BRAO. In many cases of resolved BRAO, despite the unremarkable fundus findings, the BCVA was not good. This phenomenon may be attributed to the decreased cone cells at the fovea. It can be helpful to measure the foveal thickness or ONL thickness with OCT to predict the visual prognosis in BRAO.
Figures and Tables
Fig. 1
Color fundus photographs and optical coherence tomography (OCT) image of branch retinal artery obstruction (BRAO) patient (Table 1, No. 2.). (A) Initial color fundus photograph of the left eye of a 43-year-old woman with BRAO whose best corrected visual acuity (BCVA) was 0.1. (B) Color fundus photograph of the same patient after 6 months. Her BCVA was 0.7. (C) Cross-sectional macular image with a 6-mm horizontal line scan across the central fovea. The thickness of outer nuclear laye at the central fovea is approximately 138 µm. (D) Topography image of OCT in the same patient. The average macular thickness is 179 µm, the thickness of the fovea is 221 µm. ELM=external limiting membrane; ONL=outer nuclear layer.
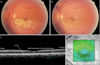
Fig. 2
Graph showing the relationship between best corrected visual acuity (BCVA) of 21 branch retinal artery obstruction eyes and outer nuclear layer (ONL) thickness, foveal thickness, average macular thickness, respectively (Spearman-rank correlation coefficient). (A) The thickness of ONL is positively correlated with BCVA (rs=0.86, p<0.001). (B) The foveal thickness is positively correlated with BCVA (rs=0.56, p=0.008). (C) There is no statically significant correlation between average macular thickness and BCVA (rs=0.41, p=0.186).

References
1. Brown GC, Shields JA. Cilioretinal arteries and retinal arterial occlusion. Arch Ophthalmol. 1979. 97:84–92.
2. Ros MA, Magargal LE, Uram M. Branch retinal-artery obstruction: a review of 201 eyes. Ann Ophthalmol. 1989. 21:103–107.
3. Brown GC, Magargal LE, Shields JA, et al. Retinal arterial obstructions in children and young adults. Ophthalmology. 1981. 88:18–25.
4. Ryan SJ, Schachat AP, Murphy RP, editors. Retina. Medical retina. 2001. Vol. 2:3rd ed. St. Louis: Mosby;1350–1367.
5. Arruga J, Sanders M. Ophthalmologic findings in 70 patients with evidence of retinal embolism. Ophthalmology. 1982. 89:1336–1347.
6. Mason JO 3rd, Shah AA, Vail RS, et al. Branch retinal artery occlusion: visual prognosis. Am J Ophthalmol. 2008. 146:455–457.
7. Schmidt D, Kube T, Feltgen N. Central retinal artery occlusion: findings in optical coherence tomography and functional correlations. Eur J Med Res. 2006. 11:250–252.
8. Hayreh SS, Zimmerman MB, Kimura A, Sanon A. Central retinal artery occlusion. Retinal survival time. Exp Eye Res. 2004. 78:723–736.
9. Leung CK, Tham CC, Mohammed S, et al. In vivo measurements of macular and nerve fibre layer thickness in retinal arterial occlusion. Eye (Lond). 2007. 21:1464–1468.
10. Asefzadeh B, Ninyo K. Longitudinal analysis of retinal changes after branch retinal artery occlusion using optical coherence tomography. Optometry. 2008. 79:85–89.
11. Hayreh SS, Podhajsky PA, Zimmerman MB. Branced retinal arterial occlusion: natural history of visual outcome. Ophthalmology. 2009. 116:1188–1194.
12. Nickla DL, Wallman J. The multifunctional choroid. Prog Retin Eye Res. 2010. 29:144–168.
13. Matsumoto H, Sato T, Kishi S. Outer nuclear layer thickness at the fovea determines visual outcomes in resolved central serous chorioretinopathy. Am J Ophthalmol. 2009. 148:105–110.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download