Abstract
Considering the popular use of antibiotic-containing eyedrops in Korea, it is important to know the emerging antibiotic-resistant strains of bacteria before treating infectious eye diseases. This is especially important in high-risk groups because of the high incidence of resistant infections and the subsequent treatment requirements. We report two cases of methicillin-resistant Staphylococcus aureus (MRSA) corneal ulcers in high-risk groups. The first case involved a patient who had keratitis after using antibiotic- and steroid-containing eyedrops to treat a corneal opacity that developed after repeated penetrating keratoplasty. The second case involved a patient who used antibiotic-containing eyedrops and a topical lubricant on a regular basis for >1 month to treat exposure keratitis due to lagophthalmos. The second patient's problems, which included a persistent superficial infiltration, developed after brain tumor surgery. Both cases showed MRSA on corneal culture, and the corneal ulcers improved in both patients after the application of vancomycin-containing eyedrops. In conclusion, MRSA infection should be considered in corneal ulcers that have a round shape, mild superficial infiltration, and slow progression, especially in high-risk groups. This report includes descriptions of the characteristic features, antibiotic sensitivities, prevention, and successful treatment with vancomycin-containing eyedrops for MRSA corneal ulcers.
Subsequent to the development of penicillin G in 1942, Staphylococcus aureus infections responded well to treatment [1]. After Kirby [2] identified the first penicillin-resistant Staphylococcus aureus in 1994, problems with resistant strains have increased. After the 1960s, methicillin showed resistance to the penicillinase that was spotlighted as a leading factor in resistance [3]. Unfortunately, as a result of the popular use of methicillin, methicillin-resistant Staphylococcus aureus (MRSA) was first reported in 1961 [4,5]. Since the late 1970s, MRSA has become a prominent infection in the hospital, especially in intensive care units (ICUs). In fact, some studies have reported that MRSA accounts for 40% to 70% of ICU staphylococcal infections [6,7].
Although MRSA does not often infect healthy individuals, it does often infect compromised hosts, including long-term inpatients, immunosuppressed patients, and patients who use antibiotics for a long time [8]. Individuals with MRSA infections are refractory to most treatments and are difficult to properly treat [9]. In 1991, Insler et al. [10] reported that fluoroquinolones were effective for treating MRSA infections. Based on that study, fluoroquinolones were selected as the first-line treatment for bacterial keratitis, and the FDA also approved them as a treatment for bacterial keratitis. In 1992 Snyder and Katz [11] reported ciprofloxacin-resistant bacterial corneal ulcers, and following that report, ciprofloxacin-resistant strains have frequently been found worldwide.
MRSA often effects high-risk patients, is easily transmitted, and acts as the source for infections in a variety of anatomic areas. Once a MRSA infection occurs, treatment can be difficult due to delayed treatment and co-existing diseases, especially in high-risk groups. Although there have been 3 case reports of MRSA ocular infections since 2002 in Korea, we describe two cases of MRSA-corneal ulcers in high-risk patients, and consider the characteristic features and methods by which to prevent future MRSA ocular infections.
A 47-year-old female presented to our institution with diminished vision on the left side and red eye for 2 months. Her past history was not significant, except for partial penetrating keratoplasty of the left eye 18 months prior to treat a corneal ulcer. She experienced one episode of graft rejection 12 months prior to presentation, and since then had been using eyedrops containing levofloxacin (Cravit; Taejoon Pharm, Seoul, Korea) and 1% rimexolone (Vexol; Alcon Laby Inc., Forth Worth, TX, USA) 4 times a day. After the rejection, a 2×2 mm, round-shaped corneal haziness associated with mild superficial infiltration had been present at the suture site in the 2 o/c area. We thought that this was a suture-related infection, so we removed the suture 6 months prior to presentation. After removal of the suture, the corneal haziness improved. In order to prevent additional rejection, we recommended the use of eyedrops containing levofloxacin and 1% rimexolone, and artificial tears 4 times a day.
When the patient visited us with the above symptoms, eyelid examination revealed a greasy appearance and meibomian gland plugs that were determined to be posterior blepharitis on the left upper and lower eyelids. On slit lamp examination, a 4×3 mm, round-shaped corneal haziness and epithelial defect associated with mild superficial stromal infiltration was found at the junction between the graft and donor site at the 2 to 3 o/c area. The cornea was edematous and the thickness was 761 µm (Fig. 1). The corneal ulcer had progressed slowly and seemed to be chronic, so we performed a corneal gram stain and culture.
We recommended warm massage and scrubbing of the upper lid and prescribed a lid cleanser (Blephasol; Samil Pharm, Seoul, Korea) in an effort to improve the eyelid hygiene. We also recommended use of eyedrops containing 5% moxifloxacin (a 4th generation fluoroquinolone) every 2 hours and 0.15% amphotericin 4 times a day, because we were unsure whether the infection was fungal or bacterial. We also recommended reducing the use of Vexol, a steroid eyedrop she had been using, to once a day. There was no improvement after 4 weeks with this treatment. Eventually, the corneal culture grew MRSA (ofloxacin resistance was also shown). According to the antibiotic sensitivity testing, we prescribed fortified 2.5% vancomycin-containing eyedrops 6 times a day. After 4 weeks, the corneal ulcer decreased to 2×3 mm in size, and we recommended reducing the use of the 2.5% vancomycin-containing eyedrops to 4 times a day. After 8 weeks, the corneal epithelium was healed completely and the corneal thickness returned to normal (581 µm) (Fig. 2).
A 62-year-old man presented with red eye, ocular pain, and eyewax on his right eye. He had a history of brain surgery to treat a brain tumor 1 year prior to presentation. After the surgery, a right-sided facial nerve palsy was noted, which reduced his blinking reflex. Postoperative exposure keratitis developed, and he began to use eyedrops containing ofloxacin (Ocuflox; Samil Pharm) and artificial tears 4 times a day.
On examination of the eyelids, a greasy appearance and meibomian gland plugs, which resembled thick toothpaste upon compressing the upper lid, were found, and posterior blepharitis involving the right upper and lower eyelids was diagnosed. On slit lamp examination, mild ciliary injection at the conjunctiva, an 8×8 mm round-shaped corneal haziness and epithelial defect associated with mild superficial stromal infiltration, and a 1.5 mm high anterior chamber hypopyon were found (Fig. 3). Gram stain and culture of the corneal tissue were performed. We recommended warm massage and scrubbing the upper lid with a lid cleanser (Blephasol) in order to improve the eyelid hygiene. We also recommended the use of eyedrops containing 5% moxifloxacin every 2 hours, alternating with 5% ceftazidime (a 3rd generation cephalosporin) every 2 hours. Additionally, we prescribed oral antibiotics. There was no improvement in the corneal ulcer or the hypopyon after 4 days of treatment. After this, as we believed the ulcer was neurotrophic and we decided to reduce the frequency of eyedrops to 6 times a day. However, after 3 days, there was no improvement. MRSA was eventually detected in the initial corneal culture, which took 1 week to grow (resistance to ofloxacin was also shown) (Fig. 4). Based on the antibiotic sensitivity testing, we prescribed fortified 2.5% vancomycin-containing eyedrops 6 times a day. After 5 days, the corneal ulcer size, superficial stromal infiltration, and hypopyon all decreased (Fig. 5). We then recommended a reduction in the use of the eyedrops containing 2.5% vancomycin to 4 times a day. By the 2 week follow-up visit, the corneal epithelium had healed completely.
The prevalence of MRSA differs depending on the group which is being studied; for example, the prevalence of MRSA was reported to be 0.1% in Geneva's Hospital [12], 2.8% in a San Francisco nosocomial community in 2000 [13], and 6.8% in a homeless group in San Francisco in 2003 [14]. The prevalence of MRSA infection is quite different among societies, but it is almost universally increasing. MRSA infections have many different appearances in different sites, and also show multiple resistances to antibiotics making treatment quite difficult. Infections in immunocompromised patients can cause severe life-threatening problems, such as necrotizing cellulitis, pneumonia, and sepsis [15]. In hospital infections, MRSA can be transmitted from patient-to-patient, via clinical materials, physician's hands, and hospital instruments. This makes preventing the spread of MRSA almost impossible [1,15-20]. Ocular MRSA infections manifest as blepharitis, conjunctivitis, keratitis, dacryocystitis, endophthalmitis, and orbital cellulitis. Generally, MRSA ocular conditions do not respond to commercially-used eyedrops, which can cause a delay in treatment and can lead to severe complications, including permanent loss of vision [21-26].
According to the study by Sotozono et al. [8], ocular MRSA infections can be divided into 4 steps. The first step is the asymptomatic carrier state or the patient who shows only conjunctivitis. The second step is the patient who shows a corneal epithelial defect with superficial infiltration. The third step is the patient who shows superficial keratitis with a corneal ulcer which progresses slowly and causes corneal stromal infiltration. The fourth step is the patient who shows corneal perforation due to severe keratitis. Thus, MRSA corneal ulcers have many manifestations, and can be treated with vancomycin-containing eyedrops or ointment according to each step, while intravenous vancomycin can be used in severe cases [8].
According to Kato and Hayasaka [27], MRSA exists as normal flora in the conjunctiva in 1.3% of the population. High-risk factors for ocular MRSA infection are known to be previous long-term use of antibiotic-containing eyedrops, long-term use of steroids, long-term hospitalization, systemic disease (respiratory failure, diabetes, cancer, and hepatic failure), ocular immunocompromised states (dry eyes, ocular surface diseases, and Stevens-Johnson syndrome), long-term use of contact lenses, and previous ocular surgery (partial penetrating keratoplasty and refractive surgery) [15,25]. Patients who have had a keratoplasty, as in case 1, have multiple risk factors. Not only do they have a history of previous ocular surgery, but they also likely have a history of long-term use of antibiotic- and steroid-containing eyedrops to prevent rejection and infection. Donor rejection must be excluded when corneal irritation symptoms develop, and consequently proper treatment can be delayed. The clinical findings of the cornea in rejection are quite different when compared to bacterial keratitis, including round superficial stromal infiltration and chronicity. Fungal keratitis should also be considered. In these situations, the use of steroid or antifungal eyedrops can aggravate the corneal ulcer and cause corneal toxicity, which can lead to severe complications, including corneal perforation. Therefore, if a patient has a slowly progressive chronic corneal ulcer and has risk factors for a MRSA infection, they should be examined and a MRSA corneal ulcer should be suspected. Corneal culture and antibiotic sensitivity testing must be performed to establish the exact diagnosis.
In patients who have been hospitalized for a long time, as in case 2, it is important to consider that MRSA could be a nosocomial infection. Nosocomial MRSA infections can cause worsening of underlying diseases, and the prevention of nosocomial MRSA spread is becoming increasingly important. Most transmission of MRSA in hospital inpatients is thought to be via transiently-colonized healthcare workers [28,29]. Environmental contamination is also a source of MRSA spread. Boyce et al. [30] found that 73% of hospital rooms with patients infected with MRSA and 69% of rooms with patients colonized with MRSA have some environmental contamination. In the same study, nurses contaminated their gloves 42% of the time in rooms with MRSA patients despite only touching environmental contamination. Other potential environmental reservoirs include computer keyboards used by clinicians [31], blood pressure cuffs, showers, and bathtubs [32]. Dietze et al. [33] found that MRSA can survive on the external surfaces of sterile goods packaged for over 38 weeks. Therefore, hand hygiene may be the single most important measure for controlling transmission of MRSA. For that reason, in MRSA patients confirmed by bacterial culture and antibiotic sensitivity testing, isolation and sterilization of the patient's medical materials and handwashing by healthcare workers are of the utmost importance [34]. The medical staff must have a thorough understanding of such policies.
Although MRSA keratoconjunctivitis can be treated with vancomycin-containing eyedrops or ointments, MRSA infections are of great concern to ophthalmologists because these ocular infections, including blepharitis, conjunctivitis, keratitis, or endophthalmitis, respond poorly to conventional antibiotic treatments and are sometimes sight-threatening. Vancomycin-containing eyedrops have several problems, including not yet being manufactured commercially. Consequently they have to be prepared directly in the hospital, which means they can be easily contaminated, may have high toxicity to the corneal epithelium, and may have unstable acidity which makes long-term storage difficult. Additionally, vancomycin-containing eyedrops might not be familiar to all ophthalmologists [35]. Unfortunately, with the high rate that bacteria are now developing antibiotic resistance, we expect that vancomycin resistant Staphylococcus will also become a problem. We should therefore choose antibiotics carefully using the results of bacterial culture and antibiotic sensitivity testing.
In corneal ulcers with a round-shaped, mild superficial infiltration that have a slow progression and in lesions that do not improve with β-lactam antibiotics, MRSA infection should be considered, especially in high-risk groups. These high-risk groups include patients who have had penetrating keratoplasty and exposure keratitis. In such cases, bacterial culture and antibiotic sensitivity testing must be performed and treatment decisions should be based on the results of these tests. Also, healthcare workers should practice serial hand washing and careful isolation of medical materials from known MRSA patients in order to prevent transmission of MRSA. The prevalence and transmission of MRSA in the community are increasing and preventing MRSA infection is becoming an increasingly important consideration.
Figures and Tables
 | Fig. 1Corneal ulcer after penetrating keratoplasty and persistent use of levofloxacin and steroid eyedrops (case 1). On slit lamp examination, a 4×3 mm round corneal opacity with a corneal epithelial defect associated with mild superficial stromal infiltration was found at the 2 to 3 o/c area around the penetrating keratoplasty stitch. Corneal edema was also noted. |
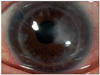 | Fig. 2Recovery of the corneal ulcer after 2 months of vancomycin eyedrops (case 1). The corneal epithelial defect with infiltration disappeared, but the corneal opacity remained. The corneal edema resolved. |
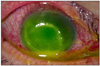 | Fig. 3Corneal ulcer after persistent use of levofloxacin eyedrops and lubricant for exposure keratitis (case 2). On slit lamp examination, an 8×8 mm round corneal ulcer with superficial stromal infiltration was found. The anterior chamber contained a 1.5 mm hypopyon associated with ciliary injection. |
Notes
References
1. Brumfitt W, Hamilton-Miller J. Methicillin-resistant Staphylococcus aureus. N Engl J Med. 1989. 320:1188–1196.
2. Kirby WM. Extraction of a highly potent penicillin inactivator from penicillin resistant staphylococci. Science. 1944. 99:452–453.
3. Rolinson GN, Stevens S, Batchelor FR, et al. Bacteriological studies on a new penicillin-BRL. 1241. Lancet. 1960. 2:564–567.
4. Jevons MP. "Celbenin"-resistant Staphylococci. Br Med J. 1961. 1:124–125.
5. Knox R. "Celbenin"-resistant Staphylococci. Br Med J. 1961. 1:126.
6. Sahm DF, Marsilio MK, Piazza G. Antimicrobial resistance in key bloodstream bacterial isolates: electronic surveillance with the Surveillance Network Database: USA. Clin Infect Dis. 1999. 29:259–263.
7. Diekema DJ, Pfaller MA, Schmitz FJ, et al. Survey of infections due to Staphylococcus species: frequency of occurrence and antimicrobial susceptibility of isolates collected in the United States, Canada, Latin America, Europe, and the Western Pacific region for the SENTRY Antimicrobial Surveillance Program, 1997-1999. Clin Infect Dis. 2001. 32:Suppl 2. S114–S132.
8. Sotozono C, Inagaki K, Fujita A, et al. Methicillin-resistant Staphylococcus aureus and methicillin-resistant Staphylococcus epidermidis infections in the cornea. Cornea. 2002. 21:S94–S101.
9. Kim IS, Shin YM, Kim SJ, et al. Comparison of antimicrobial efficacy of topical antibiotics in rabbit keratitis model with ciprofloxacin, methicillin-resistant Staphylococcus aureus. J Korean Ophthalmol Soc. 2000. 41:807–814.
10. Insler MS, Fish LA, Silbernagel J, et al. Successful treatment of methicillin-resistant Staphylococcus aureus Keratitis with topical ciprofloxacin. Ophthalmology. 1991. 98:1690–1692.
11. Snyder ME, Katz HR. Ciprofloxacin-resistant bacterial keratitis. Am J Ophthalmol. 1992. 114:336–338.
12. Harbarth S, Francois P, Shrenzel J, et al. Community-associated methicillin-resistant Staphylococcus aureus, Switzerland. Emerg Infect Dis. 2005. 11:962–965.
13. Charlebois ED, Bangsberg DR, Moss NJ, et al. Population-based community prevalence of methicillin-resistant Staphylococcus aureus in the urban poor of San Francisco. Clin Infect Dis. 2002. 34:425–433.
14. Pan ES, Diep BA, Charlebois ED, et al. Population dynamics of nasal strains of methicillin-resistant Staphylococcus aureus and their relation to community-associated disease activity. J Infect Dis. 2005. 192:811–818.
15. Blomquist PH. Methicillin-resistant Staphylococcus aureus infections of the eye and orbit (an American Ophthalmological Society thesis). Trans Am Ophthalmol Soc. 2006. 104:322–345.
16. Maple PA, Hamilton-Miller JM, Brumfitt W. World-wide antibiotic resistance in methicillin-resistant Staphylococcus aureus. Lancet. 1989. 1:537–540.
17. Brennen C, Muder RR. Conjunctivitis associated with methicillin-resistant Staphylococcus aureus in a long-term-care facility. Am J Med. 1990. 88:14N–17N.
18. Mulligan ME, Murray-Leisure KA, Ribner BS, et al. Methicillin-resistant Staphylococcus aureus: a consensus review of the microbiology, pathogenesis, and epidemiology with implications for prevention and management. Am J Med. 1993. 94:313–328.
19. Herwaldt LA. Control of methicillin-resistant Staphylococcus aureus in the hospital setting. Am J Med. 1999. 106:11S–18S.
20. Pittet D, Hugonnet S, Harbarth S, et al. Effectiveness of a hospital-wide programme to improve compliance with hand hygiene: Infection Control Programme. Lancet. 2000. 356:1307–1312.
21. Fleischer AB, Hoover DL, Khan JA, et al. Topical vancomycin formulation for methicillin-resistant Staphylococcus epidermidis blepharoconjunctivitis. Am J Ophthalmol. 1986. 101:283–287.
22. Forster W, Becker K, Hungermann D, Busse H. Methicillin-resistant Staphylococcus aureus keratitis after excimer laser photorefractive keratectomy 1. J Cataract Refract Surg. 2002. 28:722–724.
23. Goodman DF, Gottsch JD. Methicillin-resistant Staphylococcus epidermidis keratitis treated with vancomycin. Arch Ophthalmol. 1988. 106:1570–1571.
24. Khan JA, Hoover D, Ide CH. Methicillin-resistant Staphylococcus epidermidis blepharitis. Am J Ophthalmol. 1984. 98:562–565.
25. Shanmuganathan VA, Armstrong M, Buller A, Tullo AB. External ocular infections due to methicillin-resistant Staphylococcus aureus (MRSA). Eye (Lond). 2005. 19:284–291.
26. Rutar T, Chambers HF, Crawford JB, et al. Ophthalmic manifestations of infections caused by the USA300 clone of community-associated methicillin-resistant Staphylococcus aureus. Ophthalmology. 2006. 113:1455–1462.
27. Kato T, Hayasaka S. Methicillin-resistant Staphylococcus aureus and methicillin-resistant coagulase-negative staphylococci from conjunctivas of preoperative patients. Jpn J Ophthalmol. 1998. 42:461–465.
28. Mitsuda T, Arai K, Fujita S, Yokota S. Epidemiological analysis of strains of methicillin-resistant Staphylococcus aureus (MRSA) infection in the nursery: prognosis of MRSA carrier infants. J Hosp Infect. 1995. 31:123–134.
29. Cooper BS, Stone SP, Kibbler CC, et al. Isolation measures in the hospital management of methicillin resistant Staphylococcus aureus (MRSA): systematic review of the literature. BMJ. 2004. 329:533.
30. Boyce JM, Potter-Bynoe G, Chenevert C, King T. Environmental contamination due to methicillin-resistant Staphylococcus aureus: possible infection control implications. Infect Control Hosp Epidemiol. 1997. 18:622–627.
31. Devine J, Cooke RP, Wright EP. Is methicillin-resistant Staphylococcus aureus (MRSA) contamination of ward-based computer terminals a surrogate marker for nosocomial MRSA transmission and handwashing compliance? J Hosp Infect. 2001. 48:72–75.
32. Layton MC, Perez M, Heald P, Patterson JE. An outbreak of mupirocin-resistant Staphylococcus aureus on a dermatology ward associated with an environmental reservoir. Infect Control Hosp Epidemiol. 1993. 14:369–375.
33. Dietze B, Rath A, Wendt C, Martiny H. Survival of MRSA on sterile goods packaging. J Hosp Infect. 2001. 49:255–261.
34. Muto CA, Jernigan JA, Ostrowsky BE, et al. SHEA guideline for preventing nosocomial transmission of multidrug-resistant strains of Staphylococcus aureus and enterococcus. Infect Control Hosp Epidemiol. 2003. 24:362–386.
35. Suh Y, Shin EM, Hahn TW. Corneal ulcer and chronic conjunctivitis due to ofloxacin-resistant MRSA. J Korean Ophthalmol Soc. 2002. 43:419–423.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download