Abstract
Purpose
To identify the correlation between preoperative optical coherence tomography (OCT) features and postoperative visual outcomes in eyes with idiopathic macular holes (MHs).
Methods
Data from 55 eyes with idiopathic MHs which had been sealed by vitrectomy were retrospectively reviewed. Correlation analysis was conducted between postoperative visual acuity (Vpostop, logarithm of the minimum angle of resolution [logMAR]) and preoperative factors, including four OCT parameters: the anticipated length (A) devoid of photoreceptors after hole closure, MH height (B), MH size (C), and the grading (D) of the viability of detached photoreceptors. Additionally, the formula for the prediction of visual outcome was deduced.
Results
Vpostop was determined to be significantly correlated with the preoperative visual acuity (Vpreop) and OCT parameters A, C, and D (p<0.001). Based on the correlation, the formula for the prediction of Vpostop was derived from the most accurate regression analysis: Vpostop=0.248×Vpreop+1.1×10-6×A2-0.121×D+0.19.
Conclusions
The length and viability of detached photoreceptors are significant preoperative OCT features for predicting visual prognosis. This suggests that, regardless of the MH size and symptom duration, active surgical intervention should be encouraged, particularly if the MH exhibits good viability in the detached photoreceptor layer.
Although first recognized over 100 years ago, there has been renewed interest in the pathophysiology and natural history of idiopathic macular holes (MHs) over the past two decades [1]. The current surgical treatment for MHs includes complete removal of the posterior cortical vitreous, internal limiting membrane peeling, and intravitreal gas tamponade. The closure rates of idiopathic MHs have improved and have been reported to be as high as 85% to 100% [2-5]. With the development of optical coherence tomography (OCT), closure of the hole within 24 hours after vitrectomy can be demonstrated [6]. However, there exists some variability in the extent of visual recovery, even after successful MH closure [7-9]. In addition, there is some controversy regarding the predictive factors of visual outcome. Reported predictors include preoperative visual acuity, MH size [10,11] and the degree of preoperative lens opacification. Recently, several studies have shown that the postoperative thickness or defect size of the central photoreceptor was correlated with the visual prognosis [12-14]. However, thus far there have been only very limited data concerning the significance of central photoreceptor status evaluated prior to surgery for MHs.
The principal objectives of this study were to determine preoperative OCT features predictive of visual outcome after surgery for idiopathic MHs, and, based on the features, to establish a specific predictive formula.
We retrospectively reviewed the medical records of patients who underwent surgery for idiopathic MHs from August 2005 until June 2007 at the Samsung Medical Center. Institutional Review Board (IRB file number, 2009-02-045)/Ethics Committee approval was obtained. The surgeries for idiopathic MHs were conducted in 104 eyes during that period. MHs related to high myopia (≥-6.0 diopters), trauma, diabetic retinopathy, or ocular disorders that might influence vision were excluded. For inclusion in this study, the closure of the MH had to have been achieved after a single operation and have a postoperative follow-up period in excess of 6 months. If both eyes were eligible, only the eye which was operated on first was included. MH closure was confirmed via both slit lamp biomicroscopy and OCT. As such, 8 eyes (7.7%) were excluded as a consequence of primary failure and 41 eyes were excluded due to a short follow-up period. With these criteria, 55 eyes from 55 subjects were enrolled for analysis.
All patients underwent complete preoperative evaluations, including examinations for best-corrected Snellen visual acuity (BCVA), anterior segment examination, dilated fundus examination with a 90-diopter lens, and OCT (Stratus OCT ver. 3.0; Carl Zeiss Meditec, Dublin, CA, USA). In the statistical analyses, BCVA measurements were converted to the logarithm of the minimum angle of resolution (logMAR) scale. All 55 eyes had been operated on by one surgeon (SWK). The surgical procedure involved a standard three-port pars plana vitrectomy, the induction of a posterior vitreous separation with an angulated dissecting needle, the removal of the macular internal limiting membrane, and fluid-gas exchange with 14% perfluoropropane or 25% sulfur hexafluoride. In all cases, the internal limiting membranes were removed without the assistance of staining dye. The patients assumed a face-down position for 1 to 2 weeks after surgery.
There were four preoperative OCT parameters assessed (Figs. 1 and 2). The first parameter was the anticipated length (A) devoid of photoreceptors after hole closure. Parameter A was calculated by subtracting (α1+α2) from β, in which α1 and α2 were the lengths of the detached photoreceptors and β was the length of the retinal pigment epithelial layer not in contact with sensory retina. The α1, α2, and β were measured using image processing software (ImageJ; Rasband WS, US National Institutes of Health, Bethesda, MD, USA; 1997-2007). The second parameter, MH height (B), was defined as the vertical length between the retinal pigment epithelial layer and the highest portion of the MH. The third parameter, MH diameter (C), was acquired at the minimal extent of the hole. The fourth parameter was the viability (D) of the detached photoreceptors. The viability was graded from 0 to 2. Grade 2 indicated tall and healthy-looking photoreceptors with fine and regular reflectivity on OCT scans. Grade 0 was assigned to short and irregular photoreceptors with coarse reflectivity. Grade 1 was in between the two grades or used to represent an ambiguous judgment on any one section of the vertical or horizontal scans. Representative examples of the grading are provided in Fig. 2. All parameters were calculated by averaging the vertical and horizontal scan images. Preoperative OCT parameters A, B, and C were assessed by one observer masked to the visual results. Parameter D, viability of the detached photoreceptors, was assessed by three independent observers who were also masked to the visual results. In case of disagreement between observers, the grading of parameter D was decided by a majority rule. Age, gender, preoperative BCVA, duration of symptoms, and the four OCT parameters were analyzed to determine their correlation with postoperative BCVA.
From regression analysis, based on the correlation with postoperative BCVA, we deduced the formula for the prediction of the postoperative BCVA. The most accurate of many formulae was selected by the p-value of the constant. In order to validate the accuracy of the constructed formula, we applied the formula to additional cases of idiopathic MHs which had been treated by the same surgeon (20 cases from July 2007 to March 2008). The formula was also applied to cases treated at another center (20 cases treated at Asan Medical Center from June 2007 to June 2008). The surgical methods at both centers were identical, except that at the latter center indocyanine green dye was applied to stain the internal limiting membrane.
Statistical analyses were conducted using SPSS ver. 13.0 (SPSS Inc, Chicago, IL, USA). The results were considered significant at p-values of less than 0.05.
The mean (±standard deviation [SD]) age of the 55 patients was 64.7±5.8 years. There were 20 males (36.4%) and 35 females (63.6%). The postoperative follow-up periods ranged from 8 to 24 months. The mean (±SD) duration of symptoms was 3.9±3.1 months. The mean logMAR BCVA before surgery was 0.90±0.40. The mean logMAR BCVA improved to 0.33±0.30 after surgery. Serious postoperative complications were not noted.
Preoperatively, 43 eyes (78.2%) were phakic without a cataract, 10 eyes (18.2%) exhibited a mild to moderate lens opacity, and 2 eyes (3.6%) were pseudophakic. Phacoemulsification and implantation of an intraocular lens combined with vitrectomy was conducted in those 10 eyes. The development or progression of nuclear sclerotic cataracts was detected postoperatively in 27 eyes (62.8%). All of these 27 eyes were subjected to cataract surgery at 8.7±4.1 months after MH surgery.
The mean predicted length devoid of photoreceptors (A) was 172.9±154.3 µm in the horizontal scan and 168.9±141.6 µm in the vertical scan. The mean height of the MHs (B) was 662.9±146.7 µm. The mean of the initial MH diameter (C) was 402.0±151.3 µm. In the grading of the viability (D) of the detached photoreceptor layer, 13 eyes (23.6%) were classified as grade 0, 21 eyes (38.2%) as grade 1, and 21 eyes (38.2%) as grade 2. Inter-observer variability in the grading system of parameter D was identified in 12.8% of cases, in which the respective grading was decided by a majority rule. There was no case with 3 different levels of grading. In the remaining 87.2% of cases, all three observers reached complete agreement in the grading of parameter D.
The postoperative BCVA was determined to be significantly correlated with the preoperative BCVA, the predicted length devoid of photoreceptors, MH diameter, and the grading of the viability of the detached photoreceptor layer (Pearson's correlation coefficient, r=0.474, p=0.000; r=0.567, p=0.000; r=0.399, p=0.000; and r=-0.541, p=0.000, respectively). In contrast, patient age, gender, duration of symptoms, and MH height (B) were not correlated with postoperative BCVA (p=0.136, p=0.219, p=0.874, and p=0.948, respectively). The grading of photoreceptor viability in each case was analyzed by the preoperative MH diameter, a well-known prognostic factor for MH surgery (Table 1).
We noted a significant correlation between two parameters (p=0.001, chi-square test). There were only a few cases with grade 0 among the eyes with MH diameters less than 400 µm. Among the eyes with MH diameters larger than 400 µm, the eyes with grade 1 or 2 photoreceptor viability demonstrated better postoperative vision than those with grade 0 photoreceptor viability (p=0.007, Mann-Whitney test).
On the basis of the correlation, we deduced the specific formula to predict the postoperative BCVA. The following formula was derived from the most accurate regression analysis. Vpostop=0.248×Vpreop+1.1×10-6×Ah×Av-0.121×D+0.19 (Vpostop=postoperative BCVA [logMAR]; Vpreop=preoperative BCVA [logMAR]; A=anticipated length devoid of photoreceptors after hole closure [µm]; Ah=in horizontal scan; Av=vertical scan; D=viability of detached photoreceptors [the grade number]).
Although the MH diameter (C) also exhibited a correlation with the postoperative BCVA, it could not be included in the formula using the most accurate regression analysis. In the formula, the p-values that revealed the accuracy of each constant were 0.001, 0.002, 0.005, and 0.042, respectively. The discrepancy between the predicted mean BCVA and the actual postoperative mean BCVA was 0.0005±0.20 logMAR in the 55 eyes (Fig. 3A).
After applying the above formula to new groups of cases in the two study centers, the discrepancy between the predicted mean BCVA and the actual postoperative mean BCVA was 0.004±0.11 logMAR in one group (treated by the same surgeon) (Fig. 3B) and 0.05±0.17 logMAR in the other group (treated by a single surgeon at the other center) (Fig. 3C).
Analysis of MHs by OCT has provided us with a better understanding of MH geometry, prognosis, and features. Many studies have attempted to determine the prognostic values of OCT in MH surgery [12-14]. MH size measured by OCT before operation is a well-known predictor of visual prognosis [11]. Postoperatively, the anatomical closure of the MH does not always ensure a good functional result [12,13,15]. Villate et al. [14] recently emphasized that the morphology and thickness of the foveal photoreceptor layer evaluated postoperatively by OCT correlated well with macular function. However, their study evaluated the central photoreceptor status postoperatively. In addition, the majority of previous reports regarding the prognostic factors of MH surgery did not provide preoperative information for the prediction of concrete visual outcomes in individual cases.
The results of the current study strongly suggest that the preoperative length devoid of photoreceptors and the preservation of the detached photoreceptors are closely associated with the visual outcome after surgery for the treatment of idiopathic MHs. A variable extent of atrophy in the photoreceptors at the detached margin of the hole was noted in one report in which 22 postmortem eyes with idiopathic MHs were evaluated [16]. Other postmortem studies of closed MHs demonstrated that the photoreceptor inner and outer segments were relatively preserved in the perifoveal area. The central fovea was filled with glial tissues in which the photoreceptors could not regenerate, as photoreceptors grow from intact photoreceptor cell bodies [17,18]. These findings might now be studied again with OCT. Alterations in the photoreceptor layer, as evidenced by disruptions in the junction between the inner and outer segments, have been described around MHs [19,20]. We hypothesized that the preoperative OCT status of the detached photoreceptor layer might be correlated with visual prognosis in MH patients. The results of the current study showed that the length devoid of photoreceptors and the viability of the detached photoreceptor layer were significantly correlated with postoperative visual outcomes. In a previous report, microperimetry in patients with full thickness MHs demonstrated an absolute scotoma, which corresponded to the neurosensory defect, with surrounding concentric isopters of relative scotoma in the regions of the retina surrounding the hole [21]. We suspect that the anticipated length devoid of photoreceptors after hole closure should correspond to the extent of the absolute central scotoma and that the viability of detached photoreceptors should correspond to the paracentral relative scotoma. One of the principal roles of surgery for MHs seems to be the promotion of the reapproximation of the detached photoreceptors to the retinal pigment epithelium, thereby preventing the photoreceptors and their cell bodies from further degeneration. Spectral domain OCT and ultra-high resolution OCT allow for a more accurate preoperative analysis of central photoreceptor viability [20].
The diameter of the MH has been identified as a major prognostic factor for visual outcome [22,23]; this result was reproduced in this study. However, the factor of MH diameter could not be incorporated into the formula for the prediction of postoperative vision as it yielded worse predictability compared to other parameters. It is worth noting that approximately half of the cases with MHs in excess of 400 µm in diameter exhibited relatively well-preserved photoreceptor viability on preoperative OCT and that markedly better postoperative vision was observed in those eyes (Table 1). The findings of the current study suggest that surgery for the treatment of MHs should be strongly encouraged, regardless of MH size, when the detached photoreceptors are well preserved. In contrast, if the preoperative BCVA is relatively poor and the detached photoreceptors demonstrate poor viability, the patient should be preoperatively informed of the possibility of a poor visual outcome. This also highlights the essential role of early surgical intervention in MH patients, as poor viability of detached photoreceptors is anticipated in association with chronic MHs.
The limitations of the current predictive formula include the possibility of overestimating visual benefits after surgery, as only the cases in which anatomical closure was achieved were included. The predictive value would be lower if primary anatomical failure or a recurrent MH occurred in a significant proportion of eyes. However, the rate of MH sealing after the first operation was 92.3% and without recurrence in our cases, and it has been reported higher than 90% in recent studies [24-26]. Also, as we can see in additional case series, the formula could predict the postoperative BCVA within an acceptable error range, regardless of surgeon-related factors.
In conclusion, the length and the viability of the detached photoreceptor layer, as well as preoperative visual acuity, can be regarded as important prognostic factors for visual outcomes after surgery for the treatment of idiopathic MHs. The proposed formula for the prediction of postoperative vision might prove useful for preoperative counseling in such patients.
Figures and Tables
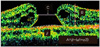 | Fig. 1Evaluated preoperative parameters in the optical coherence tomography image; calculated A refers to the anticipated length devoid of photoreceptors after closing the macular hole (MH). The length of α1, α2, and β are measured to calculate A. α1 and α2 are the curved lengths of the detached photoreceptors and β is the line length of the retinal pigment epithelial layer not in contact with the photoreceptors. MH height; B is the vertical length between the retinal pigment epithelial layer and the highest portion of the MH. MH diameter; C is determined at the minimal extent of the hole. |
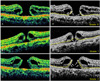 | Fig. 2Exemplary cases utilized for the grading of viability in detached photoreceptors (parameter D). Yellow dot lines indicate inner segment-outer segment junction of the photoreceptor layer; grade 2 (C,C') is assigned to tall and healthy-looking photoreceptors with fine and regular reflectivity (arrows), grade 0 (A,A') is assigned to short and irregular photoreceptors with coarse reflectivity (arrows), and grade 1 (B,B') lies in between the two grades or represents an ambiguous judgment on any one section of the vertical or horizontal scans. |
References
1. Sjaarda RN, Thompson JT. Ryan SJ, Wilkinson CP, editors. Macular hole. Retina. 2006. Vol. 3, Surgical retina:4th ed. Philadelphia: Elsevier;2527–2542.
2. Smiddy WE, Feuer W, Cordahi G. Internal limiting membrane peeling in macular hole surgery. Ophthalmology. 2001. 108:1471–1476.
3. Freeman WR, Azen SP, Kim JW, et al. The Vitrectomy for Treatment of Macular Hole Study Group. Vitrectomy for the treatment of full-thickness stage 3 or 4 macular holes: results of a multicentered randomized clinical trial. Arch Ophthalmol. 1997. 115:11–21.
4. Mester V, Kuhn F. Internal limiting membrane removal in the management of full-thickness macular holes. Am J Ophthalmol. 2000. 129:769–777.
5. Smiddy WE, Pimentel S, Williams GA. Macular hole surgery without using adjunctive additives. Ophthalmic Surg Lasers. 1997. 28:713–717.
6. Kasuga Y, Arai J, Akimoto M, Yoshimura N. Optical coherence tomograghy to confirm early closure of macular holes. Am J Ophthalmol. 2000. 130:675–676.
7. Brooks HL Jr. Macular hole surgery with and without internal limiting membrane peeling. Ophthalmology. 2000. 107:1939–1948.
8. Lai MM, Williams GA. Anatomical and visual outcomes of idiopathic macular hole surgery with internal limiting membrane removal using low-concentration indocyanine green. Retina. 2007. 27:477–482.
9. Banker AS, Freeman WR, Azen SP, Lai MY. A multicentered clinical study of serum as adjuvant therapy for surgical treatment of macular holes: Vitrectomy for Macular Hole Study Group. Arch Ophthalmol. 1999. 117:1499–1502.
10. Cheng L, Azen SP, El-Bradey MH, et al. Effects of preoperative and postoperative epiretinal membranes on macular hole closure and visual restoration. Ophthalmology. 2002. 109:1514–1520.
11. Ullrich S, Haritoglou C, Gass C, et al. Macular hole size as a prognostic factor in macular hole surgery. Br J Ophthalmol. 2002. 86:390–393.
12. Haritoglou C, Neubauer AS, Reiniger IW, et al. Long-term functional outcome of macular hole surgery correlated to optical coherence tomography measurements. Clin Experiment Ophthalmol. 2007. 35:208–213.
13. Uemoto R, Yamamoto S, Aoki T, et al. Macular configuration determined by optical coherence tomography after idiopathic macular hole surgery with or without internal limiting membrane peeling. Br J Ophthalmol. 2002. 86:1240–1242.
14. Villate N, Lee JE, Venkatraman A, Smiddy WE. Photoreceptor layer features in eyes with closed macular holes: optical coherence tomography findings and correlation with visual outcomes. Am J Ophthalmol. 2005. 139:280–289.
15. Kang SW, Ahn K, Ham DI. Types of macular hole closure and their clinical implications. Br J Ophthalmol. 2003. 87:1015–1019.
16. Guyer DR, Green WR, de Bustros S, Fine SL. Histopathologic features of idiopathic macular holes and cysts. Ophthalmology. 1990. 97:1045–1051.
17. Funata M, Wendel RT, de la Cruz Z, Green WR. Clinicopathologic study of bilateral macular holes treated with pars plana vitrectomy and gas tamponade. Retina. 1992. 12:289–298.
18. Frangieh GT, Green WR, Engel HM. A histopathologic study of macular cysts and holes. Retina. 1981. 1:311–336.
19. Srinivasan VJ, Wojtkowski M, Witkin AJ, et al. High-definition and 3-dimensional imaging of macular pathologies with high-speed ultrahigh-resolution optical coherence tomography. Ophthalmology. 2006. 113:2054.e1–2054.e14.
20. Hangai M, Ojima Y, Gotoh N, et al. Three-dimensional imaging of macular holes with high-speed optical coherence tomography. Ophthalmology. 2007. 114:763–773.
21. Sjaarda RN, Frank DA, Glaser BM, et al. Resolution of an absolute scotoma and improvement of relative scotomata after successful macular hole surgery. Am J Ophthalmol. 1993. 116:129–139.
22. Lee JE, Lee SU, Jea SY, et al. Reorganization of photoreceptor layer on optical coherence tomography concurrent with visual improvement after macular hole surgery. Korean J Ophthalmol. 2008. 22:137–142.
23. Kusuhara S, Teraoka Escano MF, Fujii S, et al. Prediction of postoperative visual outcome based on hole configuration by optical coherence tomography in eyes with idiopathic macular holes. Am J Ophthalmol. 2004. 138:709–716.
24. Smiddy WE, Glaser BM, Thompson JT, et al. Transforming growth factor-beta 2 significantly enhances the ability to flatten the rim of subretinal fluid surrounding macular holes: preliminary anatomic results of a multicenter prospective randomized study. Retina. 1993. 13:296–301.
25. Paques M, Chastang C, Mathis A, et al. Platelets in Macular Hole Surgery Group. Effect of autologous platelet concentrate in surgery for idiopathic macular hole: results of a multicenter, double-masked, randomized trial. Ophthalmology. 1999. 106:932–938.
26. Kim SS, Smiddy WE, Feuer WJ, Shi W. Outcomes of sulfur hexafluoride (SF6) versus perfluoropropane (C3F8) gas tamponade for macular hole surgery. Retina. 2008. 28:1408–1415.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download