Abstract
Purpose
To investigate the effect of catechin on apoptotic cell death in the lens epithelium of rats with cataract.
Methods
Cataract was induced by intraperitoneal injection of 100 mg/kg N-methyl-N-nitrosourea (MNU) to ten day-old Sprague-Dawley rats. The neonatal rats were randomly divided into five groups (n=15 in each group): a control group, and four cataract-induction groups, treated with either 0, 50, 100, 200 mg/kg catechin. We performed slit-lamp biomicroscopic analysis, terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) assay, Western-blot for Bcl-2 and Bax, and immunohistochemistry for caspase-3.
The lens is a unique tissue with long-lived proteins, called crystallins, which can be classified into three groups. The lens grows throughout the lifetime of an individual, and significant changes occur in the structure and function of the lens crystallins. Various modifications, such as deamidation, truncation, oxidation, glycation, and methylation, lead to structural changes in the crystallins. These mechanisms play a major role in converting the largely soluble pool of crystallins into a largely insoluble pool with aging. A cataract is an opacity that develops in the crystalline lens of the eye; it varies in degree from slight to completely opaque, obstructing the passage of light. The lens epithelium covers the anterior surface of the lens. Epithelial cells near the lens equator divide and differentiate into lens fibers. This process continues at a constant, slow rate throughout adult life, resulting in the steady growth of the lens fiber mass [1]. The mitotically quiescent central region of the epithelium is thought to protect the underlying fibers from various insults, to transport ions to and from the deeper layers of the lens, and perhaps to provide nutrients to the elongating lens fibers [2]. Damage to the lens epithelium has been a major focus in the identification of causes of cataract formation [3].
Apoptosis, also known as programmed cell death, is a form of cell death that serves to eliminate dying cells in proliferating or differentiating cell populations. Thus, apoptosis plays a crucial role in normal development and tissue homeostasis [4,5]. Previous studies have shown that apoptosis of lens epithelial cells plays an important role in the development of several types of cataracts [6-8]. These studies have suggested that apoptosis of lens epithelial cells appears as a common cellular mechanism mediating stress-induced noncongenital cataractogenesis [9,10].
Apoptosis can be detected using the terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) assay, a measure of DNA fragmentation in tissue sections, and by observation of a DNA ladder, a measure of fragmentation in DNA extracted from cells or tissues [11,12]. In human cataract research, TUNEL-positive cells indicate apoptotic cell death in the lens epithelium [1,13]. Another important characteristic of apoptosis is caspase activation. Caspase-3 is one of the most widely studied caspases, and it is a key executor of apoptosis [14]. In addition to caspases, Bcl-2 family proteins also play a pivotal role in the regulation of apoptosis. The Bcl-2 family is classified into anti-apoptotic and proapoptotic proteins according to function. The balance between pro-apoptotic and anti-apoptotic Bcl-2 family members determines the mitochondrial response to apoptotic stimuli [15].
Catechin is a naturally occurring polyphenolic compound found abundantly in green tea. Polyphenolic compounds include (-)-epgallocathechin-3-gallate (EGCG), (-)-epigallocatechin (EGC), (-)-epicatechin-3-gallate (ECG), and (-)-epicatechin (EC), the main constituents of catechin [16]. Previous studies have shown that catechin has diverse health benefits, including anti-oxidant, anti-hyperglycemic, anti-cancer, and anti-apoptotic effects [17-20]. Catechin has also been reported to exert a protective effect on UV radiation-induced epithelial cell damage of the retina [21] and lens [22].
The functional roles of catechin have been well documented, but its effects on the lens epithelium following cataract formation remain poorly understood. Although great advances have been made in surgical treatment, the incidence of cataract in developing countries is so high that it overwhelms the capacity of surgical intervention. Nonsurgical treatment alternatives are in high demand. Accordingly, we investigated the effect of catechin on apoptosis in the lens epithelium following cataract formation in rats using the TUNEL assay, Western-blot for Bcl-2 and Bax, and immunohistochemistry for caspase-3.
Neonatal Sprague-Dawley rats (seven days old) together with their maternal rats were obtained from a commercial breeder (Orient Co., Seoul, Korea). The experimental procedures were performed in accordance with the animal care guidelines of the National Institutes of Health (NIH) and the Korean Academy of Medical Sciences. Each animal was housed under controlled temperature (23±2℃) and lighting (08:00-20:00) conditions with free feeding.
The neonatal rats were randomly divided into five groups (n=15 in each group): a control group, and four cataract-induction groups, treated with either 0, 50, 100, or 200 mg/kg catechin [23,24].
The rats in the catechin-treatment groups received catechin (Sigma Chemical Co., St. Louis, MO, USA) orally once a day for ten consecutive days at the respective doses, starting five days after cataract-induction. The rats in the control group and in the cataract-induction groups received an equal amount of distilled water for the same duration.
Cataracts were induced using a previously described procedure [25]. In brief, at ten days of postnatal age, the neonatal rats received 100 mg/kg N-methyl-N-nitrosourea (MNU, Sigma Chemical Co.) intraperitoneally. Just before use, MNU was dissolved in physiological saline containing 0.05% acetic acid.
Slit-lamp biomicroscopic examination was performed on each eye to provide a morphological assessment of the degree of opacification at 15 days after cataract induction. Prior to the examination, mydriasis was achieved using a topical ophthalmic solution containing tropicamide with phenylephrine hydrochloride (Santen Pharmaceutical, Osaka, Japan). One drop of the solution was instilled in each eye every 30 minutes for 2 hours, while the animals were in a dark room. After 2 hours, the eyes were examined by slit-lamp biomicroscopy at 12×magnification.
The rats were sacrificed immediately after determination of cataract formation with slit-lamp biomicroscopy (15 days after cataract induction). The animals were anesthetized using Zoletil 50® (10 mg/kg, i.p.; Vibac Laboratories, Carros, France). At necropsy, both lenses were quickly removed under a surgical microscope GL-99B-V7 (DAVIS, California, CA, USA); a complete necropsy was performed on all animals. The lenses were fixed in 4% paraformaldehyde, dehydrated in graded ethanol, treated in xylene, and infiltrated and embedded in paraffin. Coronal sections of 5 µm thickness were made using a paraffin microtome (Leica, Nussloch, Germany) and were mounted on coated slides, then dried at 37℃ overnight on a hot plate. Six slice sections were collected on average for each lens.
The lenses were collected and immediately frozen at -70℃. The tissues were homogenized with lysis buffer containing 50 mM Tris-HCl (pH 8.0), 150 mM NaCl, 10% glycerol, 1% Triton X-100, 1.5 mM MgCl2·6H2O, 1 mM EGTA, 1 mM PMSF, 1 mM Na2VO4, and 100 mM NaF, and then ultracentrifuged at 50,000 rpm for 1 hours. Protein content were measured using a Bio-Rad colorimetric protein assay kit (Bio-Rad, Hercules, CA, USA). Forty micrograms of protein was separated on SDS-polyacrylamide gels and were transferred onto a nitrocellulose membrane. Mouse antibodies against actin (1:2000; Santa Cruz Biotech, Santa Cruz, CA, USA), Bax (1:1000; Santa Cruz Biotech), and Bcl-2 (1:1000; Santa Cruz Biotech) were used as primary antibodies. Horseradish peroxidase-conjugated anti-mouse antibodies for Bax and Bcl-2 (1:2000; Amersham Pharmacia Biothech GmbH, Freiburg, Germany) were used as secondary antibodies. The experiment was performed in normal lab conditions at room temperature, with the exception of membrane transfer. Membrane transfer was performed at 4℃ with a cold pack and a pre-chilled buffer. Band detection was performed using an enhanced chemiluminescence (ECL) detection kit (Santa Cruz Biotech). Detected bands were calculated densitometrically using Molecular Analyst™ version 1.4.1 (Bio-Rad), in order to compare the relative expressions of proteins.
TUNEL staining was performed using an In Situ Cell Death Detection Kit® (Roche, Mannheim, Germany) according to the manufacturer's protocol, in order to visualize DNA fragmentation, a marker of apoptotic cell death [26]. The epithelial cells were suspended in 10 mM Tris-HCl buffer, pH 8.0, containing 1 mM EDTA, through incubation at 55℃ for 30 minutes. Sections were then incubated with proteinase K (100 µg/ml), rinsed, incubated in 3% H2O2, permeabilized with 0.5% Triton X-100, rinsed again, and incubated in TUNEL reaction mixture. The sections were rinsed and visualized using Converter-POD with 0.03% 3,3'-diaminobenzidine (DAB) and then mounted onto gelatin-coated slides. The slides were air dried overnight at room temperature, and coverslips were mounted using Permount®.
To visualize caspase-3 expression, we performed caspase-3 immunohistochemistry using a previously described method [27]. Sections were drawn from each lens and incubated overnight with mouse anti-caspase-3 antibody (1:500; Santa Cruz Biotech) and then for another 1 h with biotinylated mouse secondary antibody (1:200; Vector Laboratories, Burlingame, CA, USA). Bound secondary antibodies were then amplified with a Vector Elite ABC Kit® (1:100; Vector Laboratories). The antibody-biotin-avidin-peroxidase complexes were visualized using 0.03% DAB, and the sections were finally mounted onto gelatin-coated slides. The slides were air dried overnight at room temperature, and coverslips were mounted using Permount®.
To compare relative expressions of proteins, we examined detected bands densitometrically using Molecular Analyst™ version 1.4.1 (Bio-Rad). The area of the lens epithelium region in each slice was measured using the Image-Pro® Plus computer-assisted image analysis system (Media Cybernetics Inc., Silver Spring, MD, USA) attached to a light microscope (Olympus, Tokyo, Japan). The TUNEL-positive and caspase-3-positive cells within each lens epithelium region were counted through the light microscope.
Statistical analysis was performed using one-way ANOVA followed by Duncan's post-hoc test. The results are expressed as mean±standard error of the mean. Significance was set at p<0.05.
The changes in lenticular opacification are presented in Fig. 1. The degree of lenticular opacification was enhanced in the cataract-induction rats and was reduced by catechin treatment.
Photomicrographs of TUNEL-positive cells in the lens epithelium are presented in Fig. 2. The number of TUNEL-positive cells was 8.30±2.78/section in the control group, 157.50±10.26/section in the cataract-induction group, 143.30±8.94/mm2 in the cataract-induction and 50 mg/kg catechin group, 136.40±11.36/section in the cataract-induction and 100 mg/kg catechin group, and 97.10±6.98/section in the cataract-induction and 200 mg/kg catechin group.
These results show that MNU injection enhanced apoptotic cell death in the lens epithelium, and catechin treatment significantly suppressed cataract-induced apoptosis in a dose-dependent manner.
Photomicrographs of caspase-3-positive cells in the lens epithelium are presented in Fig. 3. The number of caspase-3-positive cells was 6.20±2.23/section in the control group, 103.20±6.78/section in the cataract-induction group, 101.20±3.67/mm2 in the cataract-induction and 50 mg/kg catechin group, 103.00±3.10/section in the cataract-induction and 100 mg/kg catechin group, and 84.20±2.73/section in the cataract-induction and 200 mg/kg catechin group.
These results show that MNU injection enhanced caspase-3 expression in the lens epithelium, and that catechin (200 mg/kg) treatment significantly suppressed cataract-induced caspase-3 expression.
To verify cataract-induced apoptosis, we ascertained the relative protein expressions of Bax and Bcl-2 related to apoptosis, and levels of Bax and Bcl-2 are presented in Fig. 4.
The level of Bax (24 kDa, the pro-apoptotic factor) in the control group was set at 1.00. In the cataract induction group, the levels of Bax were 3.11±0.28 in the 0mg/kg catechin group, 2.79±0.28 in the 50 mg/kg catechin group, 1.98±0.18 in the 100 mg/kg catechin group, and 1.97±0.26 in the 200 mg/kg catechin group. Cataract formation increased Bax expression. In contrast, catechin administration reduced the expression of Bax in a dose-dependent manner. Specifically, 100 mg/kg and 200 mg/kg catechin significantly suppressed the expression of Bax protein.
The level of Bcl-2 (26~29 kDa, the anti-apoptotic factor) in the control group was set at 1.00. In the cataract induction groups, the levels of Bcl-2 were 1.43±0.04 in the 0mg/kg catechin group, 1.55±0.03 in the 50 mg/kg catechin group, 1.43±0.05 in the 100 mg/kg catechin group, and 1.63±0.03 in the 200 mg/kg catechin group. Cataract formation increased Bcl-2 expression, while catechin (200 mg/kg) administration slightly enhanced the expression of Bcl-2.
We calculated the ratio of Bax to Bcl-2, one of the crucial factors determining if cells will undergo apoptosis. The ratio of Bax to Bcl-2 in the control group was set at 1.00. In the cataract induction groups, the ratios of Bax to Bcl-2 were 2.17±0.20 in the 0mg/kg catechin group, 1.80±0.18 in the 50 mg/kg catechin group, 1.38±0.13 in the 100 mg/kg catechin group, and 1.21±0.16 in the 200 mg/kg catechin group.
Cataract enhanced the expression of both Bax and Bcl-2, but Bax expression increased much more than did increased Bcl-2 expression. On the other hand, administration of catechin significantly suppressed the expression of Bax protein. As a result, the ratio of Bax to Bcl-2 was increased by cataract formation, representing ongoing apoptotic cell death in the lens epithelium. Catechin treatment suppressed the ratio of Bax to Bcl-2, representing inhibition of apoptotic cell death in the lens epithelium.
Several animal species experience spontaneously occurring cataract of known inheritance and offer valuable model for studying human cataract [28]. Various chemicals are known to contribute to the development of cataract in animals. Among these chemicals, MNU, a direct-acting alkylating agent that does not require metabolic activation, is known as a cataractogenic agent in rats [29]. In addition, young animals are reported to be more susceptible to MNU than are adult animals [30]. Therefore, in this study, a cataract model was constructed using a single intraperitoneal injection of NMU in rats at postnatal day 10.
Division of the lens epithelial cells is confined to the periphery of the lens. These cells move toward the equator and then differentiate into lens fibers. Apoptosis of lens epithelial cells can occur during this differentiation process [31]. It is well known that apoptotic death of lens epithelial cells induces lens opacification. Lens epithelial cells play a vital role in the metabolic homeostasis and maintenance of transparency in the lens [32], and damage to lens epithelial cells potently contributes to cataractogenesis. Moreover, apoptosis of lens epithelial cells has been reported to be the earliest event in the experimental formation of cataracts, such as those inducted by hydrogen peroxide and MNU [32]. In human studies, the number of TUNEL-positive cells is above 50% in the lens epithelium after cataract surgery [33]. In addition, caspase-3 is up-regulated and activated in the early stages of apoptosis following cataractogenesis [31].
We found that the numbers of TUNEL-positive and caspase 3-positive cells in the lens epithelium were significantly higher following cataract induction. Opacification in the eyeball was also greater following cataract induction. These findings indicate that MNU injection-induced cataracts increased apoptosis in the lens epithelium.
The Bcl-2 family of proteins-including Bcl-2 and Bcl-xL-plays an important role in the regulation of apoptosis in the nervous system [34,35]. Bcl-2 can inhibit apoptosis by preventing the release of cytochrome-c from mitochondria. However, Bcl-2 and Bcl-xL form heterodimers with the main pro-apoptotic member Bax, which can incapacitate their protective functions [36,37]. The Bcl-2/Bax balance is one of the crucial factors determining if cells undergo apoptosis, and the balance can change during cataract formation [31].
We found that cataract formation enhanced the expressions of both Bax and Bcl-2. However, cataract formation increased Bax expression much more than it increased Bcl-2 expression.
In the lens epithelium of the cataract, lens cell death occurs by apoptosis, and inhibition of apoptosis can delay cataract formation. It has been reported that catechins can modulate apoptosis by altering the expressions of anti-apoptotic and pro-apoptotic genes [38,39]. Among the constituents of catechin, EGCG is known to protect against oxidative stress-induced and chronic glutamate-induced apoptosis in several human cells [40,41]. Yao et al [42]. reported that catechin protects against mitochondria-mediated apoptosis induced by H2O2 in human lens epithelial cells through the modulation of caspases and the MAPK and Akt pathways.
We observed that catechin significantly suppressed both cataract-induced increases in DNA fragmentation and caspase-3 expression in the lens epithelium in dose-dependent manners. In addition, catechin alleviated the degree of opacity induced by cataract formation.
Many studies have shown that EGCG, the main component of catechin, inhibits the expression of pro-apoptotic genes such as Bax, Bad, and Mdm2, and that EGCG increases the expression of anti-apoptotic genes such as Bcl-2, Bcl-w, and Bcl-xL [43,44].
In the present study, cataract enhanced the expression of both Bax and Bcl-2. However, cataracts induced apoptosis by increasing the Bax expression much more than it did the Bcl-2 expression. Administration of catechin suppressed the expression of Bax protein in a dose-dependent manner, but only slightly enhanced Bcl-2 expression. The ratio of Bax to Bcl-2 was increased by cataract formation. In contrast, catechin suppressed the ratio of Bax to Bcl-2, showing that apoptosis was inhibited by catechin treatment.
Taken together, our results demonstrate that catechin alleviated cataract-induced apoptosis in lens epithelial cells. Catechin could potentially be used to delay cataractogenesis through the suppression of apoptotic cell death in the lens epithelium.
References
1. Harocopos GJ, Alvares KM, Kolker AE, Beebe DC. Human age-related cataract and lens epithelial cell death. Invest Ophthalmol Vis Sci. 1998; 39:2696–2706. PMID: 9856780.
2. Bassnett S, Kuszak JR, Reinisch L, et al. Intercellular communication between epithelial and fiber cells of the eye lens. J Cell Sci. 1994; 107:799–811. PMID: 8056837.

3. Hightower KR, Reddan JR, McCready JP, Dziedzic DC. Lens epithelium: a primary target of UVB irradiation. Exp Eye Res. 1994; 59:557–564. PMID: 9492757.

4. Kerr JFR, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide ranging implications in tissue kinetics. Br J Cancer. 1972; 26:239–257. PMID: 4561027.
6. Lolley RN. The rd gene defect triggers programmed rod cell death. The proctor lecture. Invest Ophthalmol Vis Sci. 1994; 35:4182–4191. PMID: 8002239.
7. Papermaster DS, Windle J. Death at an early age. Apoptosis in inherited retina degenerations. Invest Ophthalmol Vis Sci. 1995; 36:977–983. PMID: 7730031.
8. Li WC. The lens epithelium, apoptosis and cataract formation. Nova Acta Leopoldina. 1997; 75:81–108.
9. Li WC, Kuszak JR, Dunn K, et al. Lens epithelial cell apoptosis appears to be a common cellular basis for non-congenital cataract development in humans and animals. J Cell Biol. 1995; 130:169–181. PMID: 7790371.

10. Li WC, Spector A. Lens epithelial cell apoptosis is an early event in the development of UVB-induced cataract. Free Radic Biol Med. 1996; 20:301–311. PMID: 8720900.

11. Gavrieli Y, Sherman Y, Ben-Sasson SA. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992; 119:493–501. PMID: 1400587.

12. Vaux DL, Strasser A. The molecular biology of apoptosis. Proc Natl Acad Sci USA. 1996; 93:2239–2244. PMID: 8637856.

13. Charakidas A, Kalogeraki A, Tsilimbaris M, et al. Lens epithelial apoptosis and cell proliferation in human age-related cortical cataract. Eur J Ophthalmol. 2005; 15:213–220. PMID: 15812762.

15. Upadhyay D, Panduri V, Ghio A, Kamp DW. Particulate matter induces alveolar epithelial cell DNA damage and apoptosis: role of free radicals and the mitochondria. Am J Respir Cell Mol Biol. 2003; 29:180–187. PMID: 12600817.
16. Bun SS, Bun H, Guédon D, et al. Effect of green tea extracts on liver functions in Wistar rats. Food Chem Toxicol. 2006; 44:1108–1113. PMID: 16487645.

17. Nakagawa T, Yokozawa T. Direct scavenging of nitric oxide and superoxide by green tea. Food Chem Toxicol. 2002; 40:1745–1750. PMID: 12419687.

18. Nagarajan S, Nagarajan R, Braunhut SJ, et al. Biocatalytically oligomerized epicatechin with potent and specific anti-proliferative activity for human breast cancer cells. Molecules. 2008; 13:2704–2716. PMID: 18978700.

19. Lim YC, Lee SH, Song MH, et al. Growth inhibition and apoptosis by (-)-epicatechin gallate are mediated by cyclin D1 suppression in head and neck squamous carcinoma cells. Eur J Cancer. 2006; 42:3260–3266. PMID: 17045795.

20. Berletch JB, Liu C, Love WK, et al. Epigenetic and genetic mechanisms contribute to telomerase inhibition by EGCG. J Cell Biochem. 2008; 103:509–519. PMID: 17570133.

21. Yang SW, Lee BR, Koh JW. Protective effects of epigallocatechin gallate after UV irradiation in cultured human retinal pigment epithelial cells. Korean J Ophthalmol. 2007; 21:232–237. PMID: 18063889.

22. Heo J, Lee BR, Koh JW. Protective effects of epigallocatechin gallate after UV irradiation of cultured human lens epithelial cells. Korean J Ophthalmol. 2007; 22:183–186. PMID: 18784447.

23. Siegers CP, Michael V, Gisela S, Younes M. Effects of dithioearb and (+)-eateehin against carbon tetrachloride-alcohol-induced liver fibrosis. Agents and Actions. 1982; 12:743–748. PMID: 6299080.
24. Anjaneyulu M, Tirkey N, Chopra K. Attenuation of cyclosporine-induced renal dysfunction by catechin: possible antioxidant mechanism. Ren Fail. 2003; 25:691–707. PMID: 14575278.

25. Kiuchi K, Yoshizawa K, Moriguchi K, Tsubura A. Rapid induction of cataract by a single intraperitoneal administration of N-methyl-N-nitrosourea in 15-day-old Sprague-Dawley (Jcl: SD) rats. Exp Toxicol Pathol. 2002; 54:181–186. PMID: 12484553.
26. Murata M, Ohta N, Sakurai S, et al. The role of aldose reductase in sugar cataract formation: aldose reductase plays a key role in lens epithelial cell death (apoptosis). Chem Biol Interact. 2001; 130-132:617–625. PMID: 11306080.

27. Sim YJ, Kim SS, Kim JY, et al. Treadmill exercise improves short-term memory by suppressing ischemia-induced apoptosis of neuronal cells in gerbils. Neurosci Lett. 2004; 372:256–261. PMID: 15542251.

28. Gelatt KN, Das ND. Animal models for inherited cataracts: a review. Curr Eye Res. 1984; 3:765–778. PMID: 6734258.

29. Roy B, Fujimoto N, Watanabe H, Ito A. Induction of cataract in methylnitrosourea treated Fischer (F344) rats. Hiroshima J Med Sci. 1989; 38:95–98. PMID: 2584061.
30. Yoshizawa K, Oishi Y, Nambu H, et al. Cataractogenesis in neonatal Sprague-Dawley rats by N-methyl-N-nitrosourea. Toxicol Pathol. 2000; 28:555–564. PMID: 10930042.
31. Lee EH, Wan XH, Song J, et al. Lens epithelial cell death and reduction of anti-apoptotic protein Bcl-2 in human anterior polar cataracts. Mol Vis. 2002; 8:235–240. PMID: 12118239.
32. Spector A. Oxidative stress-induced cataract: mechanism of action. FASEB J. 1995; 9:1173–1182. PMID: 7672510.

33. Li WC, Kuszak JR, Wang GM, et al. Calcimycin-induced lens epithelial cell apoptosis contributes to cataract formation. Exp Eye Res. 1995; 61:91–98. PMID: 7556474.

34. Yang E, Korsmeyer SJ. Molecular thanatopsis: a discourse on the BCL2 family and cell death. Blood. 1996; 88:386–401. PMID: 8695785.

35. Akhtar RS, Ness JM, Roth KA. Bcl-2 family regulation of neuronal development and neurodegeneration. Biochim Biophys Acta. 2004; 1644:189–203. PMID: 14996503.

36. Reed JC, Jurgensmeier JM, Matsuyama S. Bcl-2 family proteins and mitochondria. Biochim Biophys Acta. 1998; 10:127–137. PMID: 9714773.

37. Kuwana T, Newmeyer DD. Bcl-2-family proteins and the role of mitochondria in apoptosis. Curr Opin Cell Biol. 2003; 15:691–699. PMID: 14644193.

38. Roy AM, Baliga MS, Katiyar SK. Epigallocatechin-3-gallate induces apoptosis in estrogen receptor-negative human breast carcinoma cells via modulation in protein expression of p53 and Bax and caspase-3 activation. Mol Cancer Ther. 2005; 4:81–90. PMID: 15657356.
39. Nishikawa T, Nakajima T, Moriguchi M, et al. A green tea polyphenol, epigalocatechin-3-gallate, induces apoptosis of human hepatocellular carcinoma, possibly through inhibition of Bcl-2 family proteins. J Hepatol. 2006; 44:1074–1082. PMID: 16481065.

40. Noda C, He J, Takano T, et al. Induction of apoptosis by epigallocatechin-3-gallate in human lymphoblastoid B cells. Biochem Biophys Res Commun. 2007; 362:951–957. PMID: 17803956.

41. Tang Y, Zhao DY, Elliott S, et al. Epigallocatechin-3 gallate induces growth inhibition and apoptosis in human breast cancer cells through survivin suppression. Int J Oncol. 2007; 31:705–711. PMID: 17786300.

42. Yao K, Ye PP, Zhang L, et al. Epigallocatechin gallate protects against oxidative stress-induced mitochondria-dependent apoptosis in human lens epithelial cells. Mol Vis. 2008; 14:217–223. PMID: 18334937.
43. Levites Y, Amit T, Youdim MB, Mandel S. Involvement of protein kinase C activation and cell survival/cell cycle genes in green tea polyphenol (-)-epigallocatechin 3-gallate neuroprotective action. J Biol Chem. 2002; 277:30574–30580. PMID: 12058035.
44. Nihal M, Ahmad N, Mukhtar H, Wood GS. Anti-proliferative and proapoptotic effects of (-)-epigallocatechin-3-gallate on human melanoma: possible implications for the chemoprevention of melanoma. Int J Cancer. 2005; 114:513–521. PMID: 15609335.

Fig. 1
Slit-lamp appearance of lenses in rat eyes. (A) control group, (B) cataract-induction group, (C) cataract-induction and 200 mg/kg catechin group.
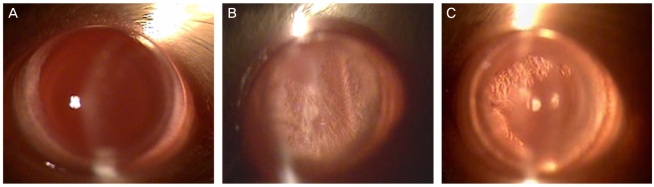
Fig. 2
Effect of catechin on DNA fragmentation in the lens epithelium induced by cataract. Upper: photomicrographs of terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL)-positive cells in the lens epithelium. (1) control group, (2) cataract-induction group, (3) cataract-induction and 200 mg/kg catechin group. The sections were stained for TUNEL. The scale bar represents 200 mm. Lower: number of TUNEL-positive cells in each group. (A) control group, (B) cataract-induction group, (C) cataract-induction and 50 mg/kg catechin group, (D) cataract-induction and 100 mg/kg catechin group, and (E) cataract-induction and 200 mg/kg catechin group.
*p<0.05 - compared to the control group; #p<0.05 - compared to the cataract-induced group.
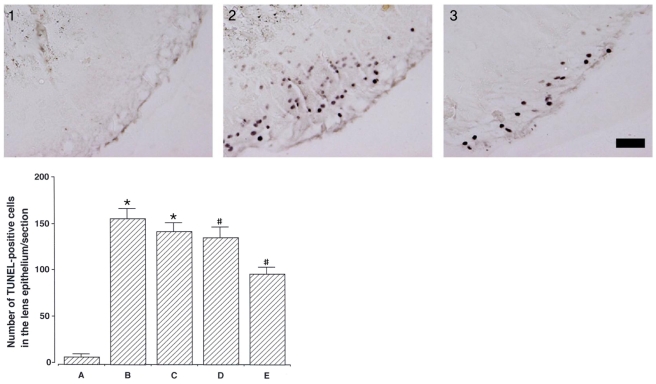
Fig. 3
Effect of catechin on caspase-3-expression in the lens epithelium induced by cataracts. Upper: photomicrographs of caspase-3-positive cells in the lens epithelium. (1) control group, (2) cataract-induction group, (3) cataract-induction and 200 mg/kg catechin group. The sections were stained for caspase-3 immunoreactivity (brown). The scale bar represents 50 mm. Lower: number of caspase-3-positive cells in each group. (A) control group, (B) cataract-induction group, (C) cataract-induction and 50 mg/kg catechin group, (D) cataract-induction and 100 mg/kg catechin group, and (E) cataract-induction and 200 mg/kg catechin group. *p<0.05 - compared to the control group; #p<0.05 - compared to the cataract-induced group.
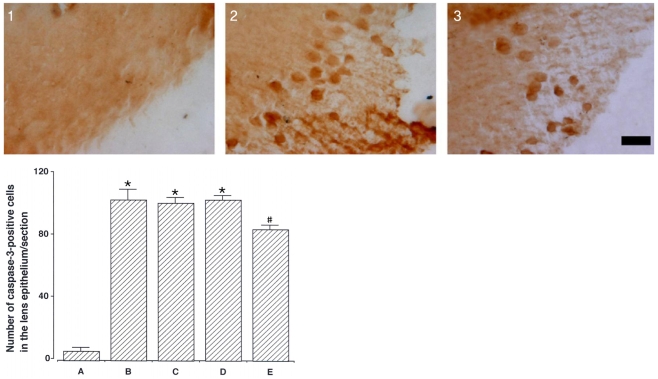
Fig. 4
Effect of catechin on the expressions of Bax and Bcl-2 proteins. Actin was used as an internal control (46 kDa). (A) control group, (B) cataract-induction group, (C) cataract-induction and 50 mg/kg catechin group, (D) cataract-induction and 100 mg/kg catechin group, and (E) cataract-induction and 200 mg/kg catechin group. Upper: the result of band detection using the enhanced chemiluminescence (ECL) detection kit. Lower: (1) The relative expression of Bax protein. (2) The relative expression of Bcl-2 protein. (3) The ratio of Bax to Bcl-2.
*p<0.05 - compared to the control group; #p<0.05 - compared to the cataract-induced group.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download