Abstract
To report a case of bilateral endophthalmitis as the initial presentation of bacterial meningitis in a young, immunocompetent Korean patient. A 35-year-old female with a one day history of bilateral swollen eyes, visual disturbance, headache, petechial skin rash, and nausea visited our clinic. She was diagnosed as having endogenous endophthalmitis associated with bacterial meningitis. Intravenous broad spectrum antibiotic therapy was initiated with cefotaxime 3 g and ubacillin 3 g, four times daily. Intravitreal antibiotic (vancomycin 1 mg/0.1 mL and ceftazidime 2 mg/0.1 mL) injections were performed in both eyes. Two weeks post presentation, the best corrected visual acuity in both eyes improved to 0.7, and inflammation of the anterior chamber and vitreous cavity was decreased. We recommend that when endogenous endophthalmitis is suspected along with meningitis, or if it is known to be present, intravitreal and intravenous antibiotics should be promptly administered to preserve vision.
Endogenous endophthalmitis is a rare, and frequently devastating, ophthalmic disease without exogenous origin. Endogenous endophthalmitis frequently occurs in patients who are immunocompromised, have diabetes mellitus, or are intravenous drug users; these categories account for over 90% of cases.1,2 Endogenous endophthalmitis in immunocompetent patients is rare, and endophthalmitis as the initial presentation of bacterial meningitis is unusual.3 A comprehensive search of the literature did not reveal any reported cases of endogenous endophthalmitis concurrent with bacterial meningitis in the Korean population. Here we describe a case of bilateral endogenous endophthalmitis as the initial presentation of bacterial meningitis in a young, immunocompetent, nondiabetic patient.
A 35-year-old Korean woman presented for medical attention due to a one day history of visual disturbance, petechial skin rash, periorbital swelling, conjunctival injection, and chemosis (Fig. 1A). She also complained of a headache and nausea. The patient had previously been healthy, was using no medication or other pharmaceutical substances, and had no ocular history of disease, trauma, or prior surgeries.
Upon examination her body temperature (39℃), blood pressure (160/100 mmHg), heart rate (82 beats/minute), and respiration rate (20/minute) were measured. The neurologic exam revealed nuchal rigidity, and laboratory studies demonstrated leukocytosis (white blood cell count: 17,890/mm3) and an elevated erythrocyte sedimentation rate (44 mm/hour).
On ophthalmic examination, the best corrected visual acuity (BCVA) was 0.1 in the right eye and hand motion in the left eye. Intraocular pressure (IOP) as measured by a Goldmann applantation tonometer was 21 mmHg in both eyes. Slit-lamp examination disclosed marked conjunctival hyperemia, corneal haziness, and small hypopyon with a fibrinous membrane covering the pupil in both eyes (Fig. 1B and 1C). Fundoscopic examination demonstrated grade II vitreous opacity in the right eye and grade IV in the left eye. Ocular motility was slightly limited in all directions.
Cerebrospinal fluid (CSF), blood, and urine samples were obtained for culture. Lumbar puncture revealed a cloudy CSF with 316 cells/mm3 of WBC (95% neutrophils), 42 mg/dL glucose (122 mg/dL serum glucose), and 120 mg/dL protein. Orbital computed tomography revealed a streaky infiltration in the retrobulbar fat and slightly thickened enhancement in the conjunctiva and preseptal spaces of both eyes (Fig. 1D).
Under the assumption of bacterial meningitis with bilateral endogenous endophthalmitis, immediate intraocular stains and cultures (anterior chamber aqueous humor 0.3 mL, vitreous 0.3 mL) and intravitreal antibiotic injection (vancomycin 1.0 mg/0.1 mL and ceftazidime 2 mg/0.1 mL) were performed. Intravenous antibiotic therapy (cefotaxime 3 g and ubacilin 3 g, four times daily) was initiated. Gram stains of intraocular samples demonstrated no microorganisms, and urine, blood, cerebrospinal fluid, and eye cultures were negative.
On day three following the intravitreal antibiotic injection, the intraocular pressure was 20 mmHg in both eyes. Inflammation of the anterior chamber, conjunctival injection, and chemosis was improved. Vitreous opacities had improved in both eyes. The intravenous and topical therapies (levofloxacin eye drops hourly and 1% atropine eye drops twice a day in both eyes) were continued for seven days.
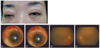
Eight days after treatment, IOP was 18 mmHg in both eyes, and the BCVA was 0.4 in the right eye and 0.2 in the left eye. Inflammation of the anterior chamber and vitreous was reduced (Fig. 2A-2C). Two weeks after initiation of therapy, the BCVA was 0.7 in both eyes. The anterior chambers were clear, and mild vitreous opacities (below grade I) were present in both eyes (Fig. 2D and 2E).
Endogenous endophthalmitis representing a metastasis from a distant focus of infection via bacteremia, accounts for about 2-8% of endophthalmitis cases, including postoperative and posttraumatic cases.4-6 Factors predisposing one to endogenous endophthalmitis are intravenous drug use, immunosuppression, and prolonged intensive care.7
This case differs from most cases of the endogenous endophthalmitis cases in that a young patient, lacking known predisposing factors, presented to ophthalmologic attention with bilateral endophthalmitis as the initial presentation of bacterial meningitis.
Cohen et al.8 reported that the most common cause of endogenous endophthalmitis was urogenital infection. Gram stains and cultures of suspect body fluids are the most important studies in evaluating and treating endogenous endophthalmitis. Greenwald et al.1 reported that the pathogen was detected 71% of the time in anterior chamber aqueous sampling and 60% of the time at vitreous sampling. However, there were many cases where neither the primary focus nor the specific pathogen was detected. In the current case, we did not identify a specific pathogen, but, the diagnosis of bacterial meningitis was verified by examination of the cerebrospinal fluid obtained by spinal tap, and the diagnosis of endogenous endophthalmitis was based on the clinical findings and ophthalmic examination.
Meningococcus is the major cause of infective meningitis, to which the younger population is most susceptible.9-13 The clinical presentations of meningococcal disease are usually acute, with fever, headache, and a petechial or purpuric skin rash being common.14 Often, meningitis presents with concurrent ocular infection, but occasionally, endophthalmitis precedes the meningitis or meningococcemia or is the only clinical presentation, without concurrent classic clinical findings of meningitis.9,14-18 A review of the literature revealed a rare case of meningococcal meningitis initially presenting as bilateral endophthalmitis.3 We assumed that the case may also be endohphthalmitis presenting with meningococcal meningitis due to the similarity of clinical presentation, especially the classic skin rash.
In reviewing previously published results, visual outcomes following endogenous endophthalmitis have been disappointing. Greenwald et al.1 reported that 40% of endogenous endophthalmitis patients achieved hand motion visual acuity, and Gonzalez et al.19 reported that 33% of cases had visual acuities of more than 0.05. Song et al.20 reported poor visual outcome in a case of endogenous enterococcus faecalis endophthalmitis in a liver abscess. Park et al.21 reported that endogenous endophthalmitis has more devastating visual outcomes than either postoperative or posttraumatic endophthalmitis, due to endogenous endophthalmitis commonly occurring in an immunocompromised host, representing more virulent pathogens, and frequently having a delayed diagnosis. In addition to intravitreal treatment, systemic antimicrobial therapy was vital for our patient, as is probably true for the majority of cases of endogenous endophthalmitis.
In summary, we reported a rare case of bilateral endogenous endophthalmitis as the initial presentation of bacterial meningitis. The patient had a good visual outcome as the result of an early diagnosis and initiation of proper treatment. This case re-emphasizes the importance of early diagnosis and treatment of endogenous endophthalmitis for good visual outcomes.
Figures and Tables
Fig. 1
At the time of admission. (A) External examination demonstrated markedly injected conjunctiva with severe chemosis and yellowish discharge in both eyes. (B, C) Slit lamp photography revealed marked conjunctival hyperemia, corneal haziness, and mild hypopyon with exudative membranes in the anterior chambers of both eyes. (D) Orbital computed tomography revealed a streaky infiltration in the retrobulbar fat and slightly thickened enhancement in the conjunctiva and preseptal spaces of both eyes.
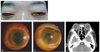
Fig. 2
Fourteen days after admission. (A) This photograph demonstrates markedly improved conjunctival injection with nearly resolved chemosis in both eyes. (B, C) Slit lamp examination also demonstrated diminished conjunctival injection and resolved chemosis and hypopyon in the anterior chambers. (D, E) Fundus photographs revealed mild vitreous opacity without focal abnormalities.
References
1. Greenwald MJ, Wohl LG, Sell CH. Metastatic bacterial endophthalmitis: a contemporary reappraisal. Surv Ophthalmol. 1986. 31:81–101.
2. Farber BP, Weinbaum DL, Dummer JS. Metastatic bacterial endophthalmitis. Arch Intern Med. 1985. 145:62–64.
3. Barnard T, Das A, Hickey S. Bilateral Endophthalmitis as an initial presentation in meningococcal meningitis. Arch Ophthalmol. 1997. 115:1472–1473.
4. Bohigian GM, Olk RJ. Factors associated with a poor visual result in endophthalmitis. Am J Ophthalmol. 1986. 101:332–341.
5. Oada AA, Johnson RP, Liles WC, et al. Endogenous bacterial endophthalmitis: report of a ten-year retrospective study. Ophthalmology. 1994. 101:832–838.
6. Puliafito CA, Baker AS, Haaf J, Foster CS. Infectious endophthalmitis: review of 36 cases. Ophthalmology. 1982. 89:921–929.
7. Ness T. Endogene endophthalmitis. Ophthalmologe. 2007. 104:935–939.
8. Cohen P, Kirshner J, Whiting G. Bacterial endogenous Escherichia coli endophthalmitis. Arch Intern Med. 1980. 140:1088–1089.
9. Wong JS, Balakrishnan V. Neisseria meningitidis endogenous endophthalmitis: case report and literature review. J Pdeiatr Ophthalmol Strabismus. 1999. 36:145–152.
10. Kaufman B, Levy H, Zaleznak BD, Litvak AM. Stastistical analysis of 242 cases of meningococcus meningitis. J Pediatr. 1951. 38:705–716.
11. Beeson PB, Westerman E. Cerebrospinal fever: analysis of 3575 case reports, with special reference to sulphonamide therapy. Br Med J. 1943. 1:497–500.
12. Jubb AA. Chemotherapy and serotherapy in cerebrospinal (meningococcal) meningitis: an analysis of 3206 case reports. Br Med J. 1943. 1:501–504.
13. Harres GE. Cerebrospinal fever: a review of 500 cases treated by chemotherapy without intrathecal serum. Br Med J. 1942. 2:423–425.
14. Bradley WG, Daroff RB, Fenichel GM, Jankovic J. Neurology in Clinical Pratice. 2008. Vol 2:5th ed. Philadelphia: Butterworth-Heinemann Elesevier;1419–1451.
15. Auerbach SB, Leach CT, Bateman BJ, et al. Meningococcal en dophthalmitis without concomitant septicemia or meningitis. Pediatr Infect Dis J. 1989. 8:411–413.
16. Kearns AM, Sprott MS. Endophthalmitis caused by Neisseria meningitides. J Infect. 1991. 22:299–300.
17. Beynon HLC, Hague S. Meningococcal endophthalmitis and pericarditis. J R Soc Med. 1990. 83:331.
18. Kerkhoff FT, van der Zee A, Bergmans AM, Rothova A. Polymerase chain reaction detection of Neisseria meningitidis in the intraocular fluid of a patient with endogenous endophthalmitis but without associated meningitis. Ophthalmology. 2003. 110:2134–2136.
19. Gonzalez GA, Whitcher JP, Irvine AR, O'Brien JM. A ring hypopyon in a patient with meningococcal endophthalmitis: a case report and review of the literature. J Pediatr Ophthalmol Strabismus. 1998. 35:329–333.
20. Song JH, Chung IY, Park JM. A case of bilateral endogenous enterococcus faecalis endophthalmitis in liver abscess. J Korean Ophthalmol Soc. 2007. 48:1291–1296.
21. Park HR, Youn YH, Sohn JH. Endogenous nocardial endophthalmitis in a renal transplant recipient. J Korean Ophthalmol Soc. 2003. 44:998–1001.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download