Abstract
Purpose
To screen for diabetic autonomic neuropathy of the pupil using 0.5% apraclonidine and 0.1% pilocarpine and to evaluate the early diagnostic value of this pharmacologic pupillary test by assessing the relationship between pupillary and cardiovascular autonomic neuropathies.
Methods
A total of 22 diabetic patients were recruited. Baseline pupillary diameter (PD) and the difference in PD between the test eye and the control eye before and after instillation of apraclonidine and pilocarpine were measured. All patients also underwent cardiovascular autonomic function (CAF) testing.
Results
Baseline PD in room light correlated with duration of diabetes mellitus (DM, p=0.049) and the presence of DM retinopathy (DMR, p=0.022). Eleven patients (50%) had positive apraclonidine tests, and two patients had positive pilocarpine tests. The patients who had positive pilocarpine tests also had positive apraclonidine tests. Patients who had a positive pupillary test had a significantly higher rate of positive CAF tests (p=0.032).
Conclusions
Pupillary autonomic neuropathy was related to the duration of diabetes and the degree of DMR. There was also a significant correlation between pupillary autonomic neuropathy and cardiovascular autonomic neuropathy (CAN). Also, sympathetic nerve dysfunction occurred prior to parasympathetic dysfunction in this study. A simple pharmacologic pupillary test can help manage complications in diabetic patients because patients with pupillary autonomic dysfunction have an increased risk of CAN.
Diabetic autonomic neuropathy (DAN) is one of the complications of both types of diabetes mellitus, developing slowly over many years.1 It can affect any part of the autonomic nervous system, including the cardiovascular, neurovascular, gastrointestinal, and genitourinary systems,2 and it can increase the mortality risk of diabetic patients.3-5 The Diabetes Complications and Control Trial6 found that intense glycemic control can prevent DAN, and other studies have shown that early-stage DAN can be reversed with intense glycemic control.7,8 Therefore, screening for autonomic dysfunction is important in the long-term care of diabetic patients. The expression pattern of DAN, however, is highly variable, and it is difficult to detect this disease on routine physical examination.
Cardiovascular autonomic neuropathy (CAN) is one manifestation of DAN that causes abnormalities in heart rate control and vascular dynamics,9 including resting tachycardia, postural hypotenics,, and exercise intolerance, and also causes an increased incindnce of silent myocardial infarction. A meta-analysis of 15 different studies cdynamdypothat the pooled estimate of myocave mortality risk was 2.14 in diabetic patients with CAN compared to subjects who had normal baseline assessments.10
The pupil is solely regulated by the autonomic nervous system. Many pharmacologic tests have been established for diagnosing various pupillary abnormalities. Apraclonidine is primarily an α2-receptor agonist that induces pupillary miosis in a normal pupil. However, apraclonidine does exhibit some weak α1-affinity11 and is known to cause mydriasis in patients with Horner's syndrome who demonstrate postsynaptic α1-receptor sympathetic denervation supersensitivity in the pupil dilator muscle due to loss of normal sympathetic innervation.11 A recent study used 0.5% apraclonidine to screen for pupillary sympathetic denervation in diabetes, identifying a defect in nearly half of the diabetic patients.12
Similarly, it is well known that the tonic pupil, which exhibits postganglionic parasympathetic denervation supersensitivity, constricts in response to 0.1% pilocarpine. Parasympathetic denervation supersensitivity has also been demonstrated in diabetic patients.13
In this study, we used 0.5% apraclonidine and 0.1% pilocarpine to screen for sympathetic and parasympathetic denervation supersensitivity, respectively, in diabetic patients in addition to evaluating the early diagnostic value of this pharmacologic pupillary test by assessing its relationship with CAN. Moreover, we investigated the correlation between the pupillary test and both the duration of diabetes and the severity of diabetic retinopathy (DMR).
A total of 22 patients with type 1 or type 2 diabetes were recruited for this study. All patients provided informed consent. Patients were excluded if they were receiving any medical treatment that influenced pupillary reaction (i.e., β-blockers, α-agonists, sympathomimetics, or cholinergics). Previous intraocular surgery history, retinal laser treatment history, and ocular pathologic conditions other than diabetic retinopathy were also exclusion criteria for this study.
At the beginning of the study, a basic ophthalmological evaluation was performed, including historical questioning regarding diabetes and other systemic conditions, visual acuity testing, a slit lamp examination, and a pupillary pharmacologic test. The details of the pupillary pharmacologic tests are as follows.
Pupil diameter (PD) was measured using a digital camera (Canon EOS 400D digital; Canon, Tokyo, Japan). Accommodation was controlled by instructing the subjects to fixate on a 3-m-distant target. Baseline PD measurements in room light (200 lux) and darkness (0.25 lux) were measured. The pharmacologic test of the parasympathetic system was performed first; 0.1% pilocarpine was instilled in one eye and 0.9% normal saline was instilled in the opposite eye as a control. The test eye was randomly selected. While waiting for an hour, patients were asked not to do any work requiring the use of near vision. PD was measured in the dark one hour after instillation. Pharmacologic testing of the sympathetic system was performed four days later to ensure complete wash out of the pilocarpine. PD was measured in the dark both before and 1 hour after instillation of 0.5% apraclonidine in the test eye and 0.9% normal saline in the control eye. Photographs were transmitted to a computer and PD was calculated. Measurements and analyses were conducted by one examiner. In order to minimize the influence of patient condition and environmental variables, the difference in PD between the test eye and the control eye before and after eyedrop instillation was used for the primary data analysis. A difference in PD of more than 1 mm was defined as a positive result for both the pilocarpine and apraclonidine tests.
After completion of the apraclonidine test, both fundi were examined with an indirect ophthalmoscope. The severity of retinopathy was classified according to the modified Early Treatment Diabetic Retinopathy Study severity scale into the following categories: no DMR, non-proliferative diabetic retinopathy (NPDR), and proliferative diabetic retinopathy (PDR).
Cardiovascular autonomic function (CAF) tests were performed to evaluate CAN. There are currently no generally accepted definitions or diagnostic methods for identifying CAN. In general, several abnormal results of various CAF tests are used as diagnostic criteria for CAN.14
We carried out four types of CAF tests, with more than two abnormal results being considered diagnostic for CAN. The tests included 1) measurement of heart rate (HR); 2) systolic blood pressure (BP) response to standing; 3) beat-to-beat heart rate variation; and 4) BP response to a Valsalva maneuver. These diagnostic tests of cardiovascular reflexes are known to be sensitive, reproducible, and simple; they also allow extensive evaluation of diabetic CAN.2
HR response to standing: The R-R interval (the time elapsing between two consecutive R waves in the electrocardiogram) was measured at beat 15 and beat 30 upon standing after 3 minutes of lying supine. A 30 : 15 ratio of less than 1.03 was considered abnormal.
Systolic BP response to standing: Systolic BP was measured when the patient was supine and again 2 minutes after standing. A fall of more than 30 mmHg was considered abnormal.
Beat-to-beat HR variation: The patient laid supine and breathed at a rate of six breaths per minute. A difference in HR of less than ten beats per minute was considered abnormal.
HR response to Valsalva maneuver: The longest R-R interval following the Valsalva maneuver and the shortest R-R interval during the maneuver defined the Valsalva ratio (VR). A VR value greater than 1.17 was considered abnormal.
We used SPSS ver. 11.0 (SPSS Inc., Chicago, IL, USA) for statistical analysis. The average PDs in room light for both positive and negative apraclonidine tests were compared using the Mann-Whitney test. The relationship between the apraclonidine test and the CAF tests was evaluated with Fisher's exact test. The relationships between PD and both the duration of diabetes mellitus (years) as well as the presence of DMR were evaluated using Spearman's rank correlation coefficient.
The mean age of the 22 patients who were included in this study was 56 years (range, 25 to 71 years), and the mean duration of diabetes was 8.96 years (range, 0 to 28 years). Six patients were receiving insulin to control blood sugar, 15 patients were taking oral hypoglycemic agents, and one patient was not taking medication. Fundus examination showed that 15 patients had no DMR, six patients had NPDR, and one patient had PDR.
There was a significant correlation between the baseline PD (mm) in room light and the duration of diabetes (years) on direct analysis (r=-0.424, p=0.049, Spearman correlation). The baseline PD in room light also correlated with the severity of DMR (r=-0.485, p=0.022, Spearman correlation) (Fig. 1).
After instillation of 0.1% apraclonidine, the mean difference in PD was 0.97 mm (range, -0.49 to 2.66 mm). Eleven patients (50%) showed differences of more than 1 mm (positive apraclonidine test). Interestingly, these patients had smaller PD measurements in room light than did the patients with negative tests (p=0.005, Mann-Whitney test). The difference in PD (mm) after 0.5% apraclonidine instillation was also significantly correlated with the presence of DMR (r=0.548, p=0.008, Spearman correlation) (Fig. 2).
The mean difference in PD (mm) after 0.1% pilocarpine instillation was -0.33 mm (range, -2.4 to 0.28 mm). Only two patients showed differences of more than 1 mm; these two patients also had positive apraclonidine tests (Fig. 3).
In CAF testing, 15 patients had positive results. Of these 15 patients, ten patients had positive apraclonidine tests. Of the seven patients who had negative CAF tests, only one patient had a positive apraclonidine test. Ten of the 11 patients who had positive pupillary tests also had positive CAF tests (Table 1). Patients who had positive apraclonidine tests had a significantly higher rate of positive results in CAF testing (p=0.032, Fisher's exact test).
The most common pupillary sign in diabetes is miosis.15 A number of previous studies have demonstrated that patients with a longer duration of diabetes have smaller pupils16-19 and that there is a statistically significant association between small pupils and the severity of retinopathy.20 We also ascertained a relationship between smaller pupils and both the duration of diabetes mellitus and presence of retinopathy in this study.
It has been suggested that failure of sympathetically-mediated pupil dilation is the mechanism of reduced pupil size in diabetics.16,21 We confirmed this using the 0.5% apraclonidine test. Eleven patients who had positive apraclonidine tests had significantly smaller pupils than did patients with negative tests. Moreover, the degree of mydriasis in diabetics correlated with the severity of DMR. In this study, we observed a high prevalence (50%) of sympathetic pupillary denervation in diabetes and a significant relationship between the pupil test and the duration of diabetes.
Several studies have suggested that sympathetic autonomic dysfunction occurs before parasympathetic dysfunction in diabetes.16,21,22 Our study confirmed this hypothesis. Of 11 patients who had positive apraclonidine tests, only two patients also had positive pilocarpine tests. Although the reason for the greater susceptibility of the sympathetic nerves is not clear, one study has suggested that it may be due to the greater lengths of the sympathetic nerve pathways.23 Histological studies have confirmed that nerve terminal loss occurs preferentially in the pupil dilator muscle, which is innervated by sympathetic nerves.15
There are conflicting reports regarding whether or not pupillary autonomic dysfunction is seen earlier than CAF.13,18,24,25 This discordance is probably due to a lack of standardization with regard to the diagnostic criteria and methods for identifying CAN. In this study, ten of the 11 patients who had positive pupillary tests also had positive CAF tests, while five out of the 15 patients with positive CAF tests had negative pupillary tests. This could imply that cardiac autonomic changes precede pupillary autonomic nervous dysfunction. Although it is not clear whether pupillary dysfunction or cardiovascular autonomic dysfunction occurs earlier, early detection of DAN is extremely important. DAN is often asymptomatic in its early stages and is one of the least recognized complications of diabetes despite its significant negative impact on survival and quality of life in diabetic patients.2
In conclusion, our study showed that pupillary autonomic neuropathy was related to the duration of diabetes and degree of DMR. Pupillary autonomic neuropathy also demonstrated a significant correlation with CAN. Additionally, sympathetic nerve dysfunction occurred prior to parasympathetic dysfunction in this study. Given that patients with pupillary autonomic dysfunction have a higher risk for developing CAN, this simple pharmacologic pupillary test can help manage the complications of diabetes.
Figures and Tables
Fig. 1
Baseline PD (mm) in room light according to severity of diabetic retinopathy (r=-0.48, p=0.022, Spearman correlation).
PD=pupillary diameter; NPDR=non-proliferative diabetic retinopathy; PDR=proliferative diabetic retinopathy.

Fig. 2
Difference in PD between test eyes and control eyes following 0.5% apraclonidine instillation according to severity of diabetic retinopathy (r=0.548, p=0.008, Spearman correlation).
PD=pupillary diameter; NPDR=non-proliferative diabetic retinopathy; PDR=proliferative diabetic retinopathy.

Fig. 3
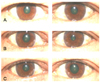
Patient who had a positive pharmacologic test. (A) Baseline photograph. (B) After instillation of 0.1% pilocarpine in the left eye and 0.9% normal saline in the right eye. (C) After instillation of 0.5% apraclonidine in the left eye and 0.9% normal saline in the right eye.
References
1. Low PA. Diabetic autonomic neuropathy. Sem Neurol. 1996. 16:143–151.
2. Vinik AI, Erbas T. Recognizing and treating diabetic autonomic neuropathy. Cleve Clin J Med. 2001. 68:928–944.
3. Ewing DJ, Boland O, Neilson JM, et al. Autonomic neuropathy, QT interval lengthening, and unexpected deaths in male diabetic patients. Diabetologia. 1991. 34:182–185.
4. Rathmann W, Ziegler D, Jahnke M, et al. Mortality in diabetic patients with cardiovascular autonomic neuropathy. Diabet Med. 1993. 10:820–824.
5. O'Brian IA, McFadden JP, Corrall RJ. The influence of autonomic neuropathy on mortality in insulin-dependent diabetes. Q J Med. 1991. 79:495–502.
6. The Diabetes Control and Complications Trial Research Group. The effect of intensive diabetes therapy on the development and progression of neuropathy. Ann Intern Med. 1995. 122:561–568.
7. Hreidarsson AB. The pupil of the eye in diabetes mellitus, an indicator of autonomic nervous dysfunction. Dan Med Bull. 1992. 39:400–408.
8. Terkildsen AB, Christensen NJ. Reversible nervous abnormalities in juvenile diabetics with recently diagnosed diabetes. Diabetologia. 1971. 7:113–117.
9. Purewal TS, Watkins PJ. Postural hypotension in diabetic neuropathy: a review. Diabet Med. 1995. 12:192–200.
10. Vinik AI, Ziegler D. Diabetic cardiovascular autonomic neuropathy. Circulation. 2007. 115:387–397.
11. Morales J, Brown SM, Abdul-Rahim AS, Crosson CE. Ocular effects of apraclonidine in Horner syndrome. Arch Ophthalmol. 2000. 118:951–954.
12. Koc F, Kansu T, Kavuncu S, Firat E. Topical apraclonidine testing discloses pupillary sympathetic denervation in diabetic patients. J Neuroophthalmol. 2006. 26:25–29.
13. Cahill M, Eustace P, de Jesus V. Pupillary autonomic denervation with increasing duration of diabetes mellitus. Br J Ophthalmology. 2001. 85:1225–1230.
14. Vinik AI, Maser RE, Mitchell BD, Freeman R. Diabetic autonomic neuropathy. Diabetes Care. 2003. 26:1553–1579.
15. Bremner FD, Smith SE. Pupil abnormalities in selected autonomic neuropathies. J Neuroophthalmol. 2006. 26:209–219.
16. Smith SE, Smith SA, Brown PM, et al. Pupillary signs in diabetic autonomic neuropathy. Br Med J. 1978. 2:924–927.
17. Hreidarsson AB. Pupil size in insulin-dependent diabetes: relationship to duration, metabolic control and long-term manifestations. Diabetes. 1982. 31:442–448.
18. Schwingshandl J, Simpson JM, Donaghue K, et al. Pupillary abnormalities in type I diabetes occurring during adolescence: comparisons with cardiovascular reflexes. Diabetes Care. 1993. 16:630–633.
19. Straub RH, Thies U, Jeron A, et al. Valid parameters for investigation of the pupillary light reflex in normal and diabetic subjects shown by factor analysis and partial correlation. Diabetologia. 1994. 37:414–419.
20. Hayashi M, Ishikawa S. Pharmacology of pupillary responses in diabetics - correlative study of the responses and grade of retinopathy. Jpn J Ophthalmol. 1979. 23:65–72.
21. Smith SA, Dewhirst RR. A simple diagnostic test for pupillary abnormality in diabetic autonomic neuropathy. Diabet Med. 1986. 3:38–41.
22. Smith SA, Smith SE. Evidence for a neuropathic aetiology in the small pupil of diabetes mellitus. Br J Ophthalmol. 1983. 67:89–93.
23. Smith SA, Smith SE. Reduced pupillary light reflex in diabetic autonomic neuropathy. Diabetologia. 1983. 24:330–332.
24. Barron SA, Rogovski Z, Kanter Y, Hemli Y. Parasympathetic autonomic neuropathy in diabetes mellitus: the heart is denervated more often than the pupil. Electromyogr Clin Neurophysiol. 1994. 34:467–469.
25. Pittasch D, Lobmann R, Behrens-Baumann W, Lehnert H. Pupil signs of sympathetic autonomic neuropathy in patients with type 1 diabetes. Diabetes Care. 2002. 25:1545–1550.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download