Abstract
Purpose
To investigate the short-term effects of panretinal photocoagulation (PRP) combined with an intravitreal injection of Avastin® (bevacizumab) as an adjuvant to high-risk proliferative diabetic retinopathy (PDR).
Methods
The data was collected retrospectively from the eyes of high-risk PDR patients, which were divided into two groups. One eye was treated with only PRP (PRP only group) and the fellow eye of same patient was treated with both PRP and intravitreal bevacizumab injection (Adjuvant group). Best corrected visual acuity (BCVA), IOP (intraocular pressure), and new vessel (NV) size in fluorescein angiography were recorded immediately and at the six-week follow-up visit. Adverse events associated with intravitreal injection were investigated.
Results
Of 12 patients with high-risk PDR, five were male and seven were female. There were no statistically significant BCVA or IOP changes after treatment in either group (p=0.916, 0.888). The reduction of NV size was found in both groups, but NV size in the adjuvant group showed a greater decrease than that of the PRP only group (p=0.038). Three patients had adverse events after intravitreal injection. Two patients had mild anterior uveitis and one patient had a serious complication of branched retinal artery obstruction (BRAO).
Conclusions
Intravitreal bevacizumab injection with PRP resulted in marked regression of neovascularization compared with PRP alone. One serious side effect, BRAO, was noted in this study. Further studies are needed to determine the effect of repeated intravitreal bevacizumab injections and the proper number of bevacizumab injections as an adjuvant.
Retinal neovascularization represents an important risk factor for severe vision loss in patients with diabetic mellitus. Proliferative diabetic retinopathy (PDR) with high-risk characteristics has a worse prognosis than in normal diabetes patients. About 30% of patients have received additional laser treatment or surgery after initial panretinal photocoagulation (PRP).1
Until now, panretinal photocoagulation (PRP) has been one of the major treatments for PDR, as it reduces the likelihood of severe vision loss caused by various complications of diabetic retinopathy.2 Immediate PRP is especially recommended when high-risk factors are involved. However, this treatment causes various adverse effects, such as increased risk of macular edema, retinal atrophy, vitreous hemorrhage and decreased peripheral vision.3,4 Furthermore, even after successful PRP, diabetic retinopathy progresses and surgical intervention may be required.1,5
Vascular endothelial factor (VEGF) has been implicated in the neovascularization of the human eye and is an important factor for the progression of PDR. Ischemic retina due to microvascular occlusion induces the release of VEGF into the vitreous cavity; highly concentrated VEGF in the ocular fluid leads to the growth of a new vessel.6 Also, VEGF increases the permeability of capillary vessels and contributes to diabetic macular edema.7,8 Recently, drugs inhibiting VEGF (bevacizumab, Avastin®; Genentech Inc., South San Francisco, CA, USA), one of the materials associated with vasculogenesis, have been developed and used. Bevacizumab (Avastin®) was originally approved for treatment of metastatic colorectal cancer in the United States.9
There have been reports indicating the effectiveness of bevacizumab on rapid regression of new vessel (NV) after a single injection, but this effect does not seem to be long-term because NV tended to recur within 12 weeks.10,11 The research herein investigated the effects of an intravitreal injection of Avastin® as an adjuvant combined with PRP in high-risk PDR patients.
A retrospective, case-controlled study was performed in the department of ophthalmology, Hanyang University Guri Hospital. Medical records of 12 patients who were diagnosed with first-time high-risk PDR in both eyes and who were treated with PRP with an intravitreal injection of bevacizumab in one eye and single PRP therapy in the other eye were reviewed for this study. The patient data was collected from May 2007 to May 2008. None of the patients had ever received any prior therapy before the first visit. We divided all study eyes into two groups. One group, defined as the control group, included eyes managed by single laser therapy. Another group, defined as the treatment group, consisted of eyes treated with laser therapy combined with a single adjuvant intravitreal bevacizumab injection. High-risk PDR was defined by Early Treatment Diabetic Retinopathy Study Research Group (ETDRS) guidelines.12 Patients who had the following risk factors were assigned to the high-risk PDR group. 1) Presence of neovascularization of disc (NVD) >ETDRS standard photograph 10A; 2) less extensive NVD, if vitreous or pre-retinal hemorrhaging was present, 3) NV of elsewhere (NVE) ≥ 1/2 disc area, if vitreous or pre-retinal hemorrhaging was present. Exclusion criteria included 1) history of previous laser treatment, vitreoretinal surgery, or intravitreal injection; 2) history of another ocular disease other than PDR. Ophthalmologic evaluations were performed, including anterior segment examination, logMAR best corrected visual acuity (BCVA), IOP measurement and fundus examination for baseline and follow-up data. Fundus photography and fluorescein angiography (FAG) were taken before the first PRP and at the six-week follow-up visit.
Data for 12 patients was reviewed. Both panretinal photocoagulation and intravitreal Avastin® injection (treatment group) were performed for one eye, and only panretinal photocoagulation (PRP only group) was conducted on the fellow eye of same patient. Intravitreal Avastin® injection was done after the first laser treatment using the following method: 1) the area around the eye was sterilized with 5% povidone/iodine, 2) intravitreal Avastin® 0.05 mL (1.25 mg) was administered using a tuberculin syringe with a 30 G needle, 3) antibiotic eye drops and oral antibiotics were prescribed for one week after the treatment.
All injections were performed in the operating room. Patients visited an outpatient clinic for the examination of visual acuity, intraocular pressure, anterior segment, and fundus the day after treatment. The panretinal photocoagulation protocol followed Diabetic Retinopathy Study Research Group guidelines. Before PRP, topical anesthesia using 0.5% proparacaine (Alcaine®; Alcon Laboratories, Hunenberg, Switzerland) was dropped. Three hundred to four hundred argon laser (514 nm) burns with a spot size of 500 µm were made each time (800-1,600 burns in total) using a fundus contact lens (Transequator®; Volk Optical, Mentor, OH, USA). Panretinal photocoagulation was executed in the inferior, nasal, superior, and temporal areas in both eyes, one each week over a two-week time period.
The differences in the two sets of eyes at 4-8 weeks after treatment were compared and analyzed using the fluorescein angiography image analysis program (Image J®, Rasband, W.S. Image J ver. 1.36b; U.S. National Institutes of Health, Bethesda, MD, USA; http://rsb.info.nih.gov/ij/, 1977-2006). Also, BCVA, IOP, anterior segment results, and fundus status were compared. Adverse effects associated with intravitreal bevacizumab injection were evaluated, including BCVA (logMAR), size and activity of neovascularization on fundus angiography compared with baseline angiography, and intraocular pressure change.
For image analysis, only the central views (50 degrees) of the angiographic images including NVDs were chosen in the mid-phase of FAG. Peripheral images were excluded because the size of same image was different by photographing angle. In the presence of early NVD, it was difficult to outline the image, so another NVE prominent in the central view was selected. After choosing the images, the examiner outlined the area of NVD using the contrast enhancement tool Image J®. The NV size was expressed in pixels. The Wilcoxon signed ranks test was used for statistical analysis with SPSS ver. 13.0 (SPSS Inc., Chicago, IL, USA).
Of the 12 patients with high-risk PDR, five were male and seven were female. The mean age was 50.45±3.30 years, and the mean diabetes duration was 9.00±1.95 years. All patients involved in this study had type 2 diabetes mellitus. Eleven patients remained to take a follow-up FAG examination six weeks after treatment (Tables 1 and 2) One patient was lost to follow-up. The baseline mean BCVA was 0.32±0.11 in the PRP only eyes and was 0.25±0.09 in the Avastin® adjuvant eyes. After treatment, mean BCVA was 0.28±0.10 in the PRP only eyes and 0.24±0.11 in the Avastin® adjuvant eyes (Tables 1 and 2). In both groups, there were no statistically significant BCVA changes after treatment (p=0.493, 0.705, respectively). No clinically significant macular edema was observed in any of the patients. Intraocular pressure was similar before and after treatment in both groups (p=0.574, 0.310, respectively). Follow-up FAG examination showed the reductions in NV size in both groups to be statistically significant (p=0.008, 0.005, respectively). The baseline area of NV was 12.33±3.82 pixels (×103) in the PRP only group and 17.16±3.14 pixels (×103) in the Avastin® adjuvant group. The follow-up NV results were 7.69±3.54 pixels (×103) in the PRP only group and 5.65±2.27 pixels (×103) in the Avastin® adjuvant group (Table 3). Neovascularization size in the adjuvant group revealed more regression than did that of the PRP only group. (p=0.038) (Fig. 1). Clinically, based on FAG interpretation by a retinal specialist, all Avastin® adjuvant eyes showed marked reduction in NV size and activity. In the PRP only group, NV size decreased but activity still remained in some patients (Fig. 2).
Disease progression was found in two patients. Both patients had vitreous hemorrhages in the PRP only eye. The vitreous hemorrhages were not absorbed spontaneously, so vitrectomies were performed for both patients. Three patients had adverse events associated with intravitreal bevacizumab injection. Two had mild anterior uveitis that occurred one day after injection. These patients were prescribed antibiotic eye drops for seven days. No definite cell in the anterior chamber was found after one week in either patient. One patient suffered a branched retinal artery obstruction observed one month after the second injection (Fig. 3). This patient experienced amaurosis fugax-like symptoms in her left eye the day before the follow-up appointment. Her BCVA was decreased from 0.1 to 0.03. She received anticoagulant therapy after admission, but her visual acuity in the involved eye was not recovered.
Panretinal photocoagulation is a well-known principal therapy for proliferative diabetic retinopathy.2 Although PRP reduces the possibility of severe visual loss, it has several side effects, such as macular edema, constricted visual field and laser-induced vitreous hemorrhage.3,4 Also, additional laser therapy or surgical intervention has been necessary after PRP performance. Recent reports have shown that VEGF plays a key role in neovascularization of the eye, and that intravitreal anti-VEGF injection can lead to regression of NV in neovascular age-related macular degeneration, central retinal vein obstruction, iris neovascularization and proliferative diabetic retinopathy.6-8,10,11,13-15 Ehlers et al.16 discovered the combination intravitreal bevacizumab/PRP effect for treatment of neovascular glaucoma that rapidly decreases NV and IOP.
Avery et al.10 reported that rapid regressions in retinal and iris NV were shown after intravitreal bevacizumab injection. However, the recurrence of NV was noted in one patient. Another study by Jorge et al.11 also showed the recurrence of NV leakage 12 weeks after injection. In this current study, we researched the difference in the regression and activity of NV between PRP with a single Avastin® injection and with PRP alone in high-risk PDR patients. Marked regression of NV was observed in the Avastin® adjuvant group compared with that of the PRP only group. Some eyes in the PRP only group developed new vitreous hemorrhages and vitrectomies were performed. It was thought that high-risk PDR patients were more vulnerable to destructive therapy like PRP that may induce vitreous hemorrhage. In this study, the effect of NV regression in the PRP only group had statistical significance (p=0.005). However, size and activity of the NV were more decreased in the Avastin® adjuvant group, as confirmed with an FAG exam. The results were statistically significant (p=0.038).
Tonello et al.17 also showed that the adjuvant use of intravitreal bevacizumab injection with PRP induced greater NV regression than did PRP alone in high-risk PDR patients in short-term observation. Similarly, Mirshahi et al.18 evaluated the effect of bevacizumab-augmented retinal laser photocoagulation in high-risk PDR patients. They found that the combination therapy had a more effective response in the regression of NV at six weeks of follow-up. However, PDR recurred at week 16 of follow-up in the bevacizumab-injected eyes, and the complete regression rate was similar to that of the PRP alone group. We were unable to determine whether adjuvant therapy inhibits the recurrence of NV or maintains a long-term remission state. Best corrected visual acuity was unchanged in both groups after treatment. These results were similar to those of other studies.17 In our study, no significant macular edema was observed in either group during the study period. Macular edema is the leading cause of visual loss in diabetic retinopathy patients.19 Because there was no development of clinically significant macular edema, it was thought that BCVA in the two groups was similar. However, when considering the possibility of PRP-induced macular edema, if a larger number of cases were included in the study, the BCVA of the Avastin® adjuvant group might have been superior to that of the PRP only group because bevacizumab has been shown to reduce macular edema in diabetic retinopathy.20,21 Intraocular pressure changes were also not different between the two groups. Similar results were found in the literature.11,17 Many reports showed that the injection of intravitreal bevacizumab was well-tolerated and safe. However, there was intraocular inflammation in two cases and one serious complication (branched retinal artery obstruction, BRAO) after intravitreal bevacizumab injection in this study. In the literature, some studies mentioned complications related to intravitreal bevacizumab injection. Bakri et al.22 reported four occurrences of self-limited sterile uveitis after injection and our uveitis patients also had similar courses. Shima et al.23 demonstrated various complications associated with intravitreal injection of bevacizumab in 707 patients; not only intraocular complications but also extraocular complications, such as cerebral infarction, were shown. There were other studies highlighting arterial thromboembolic adverse events, including myocardial infarction and cerebrovascular accident, in the treatment of metastatic colon cancer with bevacizumab.24,25 In our case, BRAO may have occurred due to the thromboembolic effects of bevacizumab.
In conclusion, intravitreal bevacizumab injection with PRP resulted in marked regression of neovascularization and decreased the risk concerning newly developed vitreous hemorrhage and rapidly progressing fibrovascular proliferation. Recently, many medical centers have begun to make attempts to apply intravitreal bevacizumab injections to various eye diseases, based on the successful results of previous studies. However, we experienced unfavorable adverse events after injection of bevacizumab. Therefore, intravitreal bevacizumab injection should be performed with caution until its long-term effects and safety are confirmed in future studies. A small sample size and short duration of follow-up were limitations of this study. Also, in the estimation of NV regression, it was difficult to outline the NV area in FAG correctly, and errors were possible with respect to the assessor's subjectivity. Further studies are needed to determine the effects of repeated intravitreal bevacizumab injections and the proportions of adjuvant bevacizumab injection with PRP to reduce the risk of new vitreous hemorrhages and macular edema during treatment.
Figures and Tables
Fig. 1
New vessel (NV) size change at baseline and the follow-up visit. The NV regression of the treatment group is steeper than that of the control group. The result was statistically significant (p=0.038). C=control (panretinal photocoagulation only group); T=treatment (adjuvant group).

Fig. 2
Case 3. A 51-yr-old female patient with bilateral high-risk proliferative diabetic retinopathy. (A, B) Actively leaking new vessels were observed in both eyes at baseline. She received the standard panretinal photocoagulation in both eyes. Intravireal bevacizumab injection was added to the treatment of her right eye. (C) At eight weeks, a marked decrease of leakage was noted in the right eye. (D) In her left eye, leakage from NV was slightly decreased but still actively persistent.
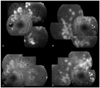
Fig. 3
Case 1. A 53-yr-old female patient. (A) Neovascularization (NV) of the disc and a pre-retinal hemorrhage were seen at her first visit. Visual acuity in her left eye was 0.4. (B) After an intravitreal injection of bevacizumab, NV size decreased but persisted at six wk. Therefore, a second injection was performed. She complained of sudden visual loss and amaurosis at two wk after the second injection in her left eye. She visited our clinic 30 hr later with a visual acuity of 0.03. (C) A cherry red spot and retinal opacification were observed. Retinal arterial occlusion was diagnosed.
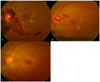
Table 1
Clinical characteristics of the 12 patients enrolled in the current study

Vitreous hemorrhage (VH) occurred in the control group in the eyes of cases 7 and 8.
The case 8 patient experienced branched retinal artery obstruction. Case 12 was a follow-up loss.
BCVA (logMAR)=best corrected visual acuity; IOP=intraocular pressure; NV=neovascularization; OD=right eye; OS=left eye.
*The eye in which intravitreal Avastin® was injected; †Treatment group=Avastin® adjuvant therapy; ‡Control group=panretinal photocoagulation only therapy.
References
1. Kaiser RS, Maguire MG, Grunwald JE, et al. One-year outcomes after panretinal photocoagulation in proliferative diabetic retinopathy. Am J Ophthalmol. 2000. 129:178–185.
2. Early photocoagulation for diabetic retinopathy: ETDRS report number 9. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology. 1991. 98:5 Suppl. 766–785.
3. Kleiner RC, Elman MJ, Murphy RP, Ferris FL 3rd. Transient severe visual loss after panretinal photocoagulation. Am J Ophthalmol. 1988. 106:298–306.
4. McDonald HR, Schatz H. Macular edema following panretinal photocoagulation. Retina. 1985. 5:5–10.
5. Flynn HW Jr, Chew EY, Simons BD, et al. Pars plana vitrectomy in the Early Treatment Diabetic Retinopathy Study. EDTRS report number 17. The Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology. 1992. 99:1351–1357.
6. Aiello LP, Avery RL, Arrigg PG, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994. 331:1480–1487.
7. Murata T, Nakagawa K, Khalil A, et al. The relation between expression of vascular endothelial growth factor and breakdown of the blood-retinal barrier in diabetic rat retinas. Lab Invest. 1996. 74:819–825.
8. Funatsu H, Yamashita H, Noma H, et al. Aqueous humor levels of cytokines are related to vitreous levels and progression of diabetic retinopathy in diabetic patients. Graefes Arch Clin Exp Ophthalmol. 2005. 243:3–8.
9. Yang JC, Haworth L, Sherry RM, et al. A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic rectal cancer. N Engl J Med. 2003. 349:427–434.
10. Avery RL, Pearlman J, Pieramici DJ, et al. Intravitreal bevacizumab (Avastin) in the treatment of proliferative diabetic retinopathy. Ophthalmology. 2006. 113:1695–1705.
11. Jorge R, Costa RA, Calucci D, et al. Intravitreal bevacizumab (Avastin) for persistent new vessels in diabetic retinopathy (IBEPE study). Retina. 2006. 26:1006–1013.
12. Grading diabetic retinopathy from stereoscopic color fundus photographs: an extension of the modified Airlie House classification. ETDRS Report No. 10. Ophthalmology. 1991. 98:5 Suppl. 786–806.
13. Rosenfeld PJ, Moshfeghi AA, Puliafito CA. Optical coherence tomography findings after an intravitreal injection of bevacizumab (Avastin) for neovascular age-related macular degeneration. Ophthalmic Surg Lasers Imaging. 2005. 36:331–335.
14. Rosenfeld PJ, Moshfeghi AA, Puliafito CA. Optical coherence tomography findings after an intravitreal injection of bevacizumab (Avastin) for macular edema from central retinal vein occlusion. Ophthalmic Surg Lasers Imaging. 2005. 36:336–339.
15. Oshima Y, Sakaguchi H, Gomi F, Tano Y. Regression of iris neovascularization after intravitreal injection of bevacizumab in patients with proliferative diabetic retinopathy. Am J Ophthalmol. 2006. 142:155–158.
16. Ehlers JP, Spirn MJ, Lam A, et al. Combination intravitreal bevacizumab/panretinal photocoagulation versus panretinal photocoagulation alone in the treatment of neovascular glaucoma. Retina. 2008. 28:696–702.
17. Tonello M, Coast TA, Almeida FP, et al. Panretinal photocoagulation versus PRP plus intravitreal bevacizumab for high-risk proliferative diabetic retinopathy (IBeHi study). Acta Ophthamol. 2008. 86:385–389.
18. Mirshahi A, Roohipoor R, Lashay A, et al. Bevacizumab-augmented retinal laser photocoagulation in proliferative diabetic retinopathy: a randomized double-masked clinical trial. Eur J Ophthalmol. 2008. 18:263–269.
19. Aiello LM. Perspectives on diabetic retinopathy. Am J Ophthalmol. 2003. 136:122–135.
20. Soheilian M, Ramezani A, Bijanzadeh B, et al. Intravitreal bevacizumab (avastin) injection alone or combined with triamcinolone versus macular photocoagulation as primary treatment of diabetic macular edema. Retina. 2007. 27:1187–1195.
21. Haritoglou C, Kook D, Neubauer A, et al. Intravitreal bevacizumab (Avastin) therapy for persistent diffuse diabetic macular edema. Retina. 2006. 26:999–1005.
22. Bakri SJ, Larson TA, Edwards AO. Intraocular inflammation following intravitreal injection of bevacizumab. Graefes Arch Clin Exp Ophthalmol. 2008. 246:779–781.
23. Shima C, Sakaguchi H, Gomi F, et al. Complications in patients after intravitreal injection of bevacizumab. Acta Ophthalmologica. 2008. 86:372–376.
24. Scappaticci FA, Skillings JR, Holden SN, et al. Arterial thromboembolic events in patients with metastatic carcinoma treated with chemotherapy and bevacizumab. J Natl Cancer Inst. 2007. 99:1232–1239.
25. Hurwitz H, Saini S. Bevacizumab in the treatment of metastatic colorectal cancer: safety profile and management of adverse events. Semin Oncol. 2006. 33:S26–S34.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download