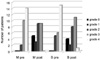
1. Scott AB, Kennedy RA, Stubbs HA. Botulinum A toxin injection as a treatment for blepharospasm. Arch Ophthalmol. 1985. 103:347–350.
2. Jankovic J, Orman J. Botulinum A toxin for cranial-cervical dystonia: a double-blind, placebo-controlled study. Neurology. 1987. 37:616–623.
3. Taylor JD, Kraft SP, Kazdan MS, et al. Treatment of blepharospasm and hemifacial spasm with botulinum A toxin: a Canadian multicentre study. Can J Ophthalmol. 1991. 26:133–138.
4. Park YC, Lim JK, Lee DK, Yi SD. Botulinum a toxin treatment of hemifacial spasm and blepharospasm. J Korean Med Sci. 1993. 8:334–340.
5. Elston JS. Long-term results of treatment of idiopathic blepharospasm with botulinum toxin injections. Br J Ophthalmol. 1987. 71:664–668.
6. Sampaio C, Ferreira JJ, Simoes F, et al. DYSBOT: a single-blind, randomized parallel study to determine whether any differences can bedetected in the efficacy and tolerability of two formulations of botulinum toxin type A--Dysport and Botox--assuming a ratio of 4:1. Mov Disord. 1997. 12:1013–1018.
7. Nussgens Z, Roggenkamper P. Comparison of two botulinum-toxin preparations in the treatment of essential blepharospasm. Graefes Arch Clin Exp Ophthalmol. 1997. 235:197–199.
8. Truong D, Comella C, Fernandez HH, et al. Efficacy and safety of purified botulinum toxin type A (Dysport) for the treatment of benign essential blepharospasm: a randomized, placebo-controlled, phase II trial. Parkinsonism Relat Disord. 2008. 14:407–414.
9. Roggenkamper P, Jost WH, Bihari K, et al. Efficacy and safety of a new Botulinum Toxin Type A free of complexing proteins in the treatment of blepharospasm. J Neural Transm. 2006. 113:303–312.
10. Rieder CR, Schestatsky P, Socal MP, et al. A double-blind, randomized, crossover study of prosigne versus botox in patients with blepharospasm and hemifacial spasm. Clin Neuropharmacol. 2007. 30:39–42.
11. Cakmur R, Ozturk V, Uzunel F, et al. Comparison of preseptal and pretarsal injections of botulinum toxin in the treatment of blepharospasm and hemifacial spasm. J Neurol. 2002. 249:64–68.
12. Stone AV, Ma J, Whitlock PW, et al. Effects of Botox and Neuronox on muscle force generation in mice. J Orthop Res. 2007. 25:1658–1664.
13. Khoo HM, Kim JC, Khoo BS. Treatment of blepharospasm and hemifacial spasm with Botulinum toxin A (Oculinum®). J Korean Ophthalmol Soc. 1990. 31:59–68.
14. Kim JC, Kim WS, Ahn SK, Shyn KH. Clinical studies in patients with essential blepharospasm and with hemifacial spasm. J Korean Ophthalmol Soc. 1991. 32:837–843.
15. Hatheway CH, Snyder JD, Seals JE, et al. Antitoxin levels in botulism patients treated with trivalent equine botulism antitoxin to toxin types A, B, and E. J Infect Dis. 1984. 150:407–412.
16. Cote TR, Mohan AK, Polder JA, et al. Botulinum toxin type A injections: adverse events reported to the US Food and Drug Administration in therapeutic and cosmetic cases. J Am Acad Dermatol. 2005. 53:407–415.
17. Kenney C, Jankovic J. Botulinum toxin in the treatment of blepharospasm and hemifacial spasm. J Neural Transm. 2008. 115:585–591.
18. Greene P, Fahn S, Diamond B. Development of resistance to botulinum toxin type A in patients with torticollis. Mov Disord. 1994. 9:213–217.







 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download