Abstract
Purpose
Methods
Results
Conclusions
Figures and Tables
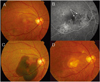 | Fig. 1Case 4, with exudative maculopathy in the right eye. (A) Fundus photograph showing subretinal blood and subretinal serous detachment. (B) Midphase fluorescein angiogram (FA) showing an irregular hyperfluorescent area corresponding to the interconnecting vascular network (arrow). (C) Fundus photograph 4 weeks after photodynamic therapy (PDT) showing a massive subretinal hemorrhage, including the posterior pole. (D) Fundus photograph after two intravitreal bevacizumab injections followed by an intravitreal injection of tissue plasminogen activator (t-PA) and 100% C3F8 (0.3 ml), showing chorioretinal scarring. |
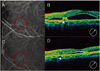 | Fig. 2Case 3, left eye, the fellow eye of the case 4 patient (Fig. 1). (A) Midphase indocyanine green angiogram (ICGA), showing multiple polypoidal dilations of choroidal vessels and an interconnecting network. (B) Optical coherence tomograph (OCT) showing subfoveal serous elevation and a polypoidal lesion (arrow head). (C) Midphase ICGA showing reduced polypoidal lesion and interconnecting vessels. (D) OCT after three intravitreal bevacizumab injections, showing reduced subretinal fluid and a polypoidal lesion re-attached to the Bruch's membrane and choroids (arrow head). |
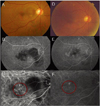 | Fig. 3Case 8 with persistent active polypoidal choroidal vasculopathy (PCV) 4 months after photodynamic therapy (PDT). (A) Fundus photograph showing dense subretinal hemorrhages and serous subretinal detachment. (B) Midphase fluorescein angiogram (FA) showing hypofluorescence corresponding to a subretinal hemorrhage and multiple dot-shaped hyperfluorescent lesions. (C) Midphase indocyanine green angiogram (ICGA) showing multiple polypoidal dilations of choroidal vessels and an interconnecting vascular network. (D) Fundus photograph after three intravitreal bevacizumab injections, showing resolved subretinal hemorrhages and serous subretinal detachment. (E) Midphase FA after three intravitreal bevacizumab injections, showing only the retinal pigment epithelium (RPE) window defect without angiographic leakage in late phase (not shown). (F) Midphase ICGA after three intravitreal bevacizumab injections, showing regression of the polyps and an interconnecting vascular network. |
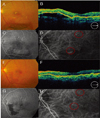 | Fig. 4Case 12 (A) Fundus photograph showing some hemorrhages, subretinal serous detachment, and subretinal exudates. (B) Optical coherence tomograph (OCT) showing serous pigment epithelial detachment (PED). (C) Midphase fluorescein angiogram (FA) showing hyperfluorescence with serous PED and round, multiple isolated hyperfluorescence lesions and surrounding irregular hyperfluorescence. (D) Midphase indocyanine green angiogram (ICGA) showing multiple polypoidal dilation of the choroidal vessels and interconnecting network. (E) Fundus photograph after three intravitreal bevacizumab injections and one round of photodynamic therapy (PDT), showing reduced hemorrhages and subretinal serous elevation. (F) OCT after three intravitreal bevacizumab injections and one round of PDT, showing resolved serous PED. (G) Midphase fluorescein angiogram (FA) after three intravitreal bevacizumab injections and one round of PDT, showing reduced hyperfluorescence and reduced subretinal fluid. (H) Midphase ICGA after three intravitreal bevacizumab injections and one round of PDT, showing resolved polypoidal lesion and interconnecting vessels. |
Table 1

M=male; F=female; Tx=treatment; F/U=follow-up; P=photodynamic therapy, PDT; T/G=t-PA with gas injection; L=focal laser photocoagulation, A=intravitreal bevacizumab; BCVA=best corrected visual acuity; PED=pigment epithelial detachment; SRF=subretinal fluid;RPE=retinal pigment epithelium.
*Treatment (time, weeks); **BCVA before intravitreal bevacizumab; †follow-up duration after intravitreal bevacizumab injection; ‡Localized bleeding at 6 weeks after PDT plus intravitreal bevacizumab.
Table 2

M=male; F=female; Tx=treatment; F/U=follow-up; P=photodynamic therapy, PDT; T/G=t-PA with gas injection; A=intravitreal bevacizumab; BCVA=best corrected visual acuity; PED=pigment epithelial detachment; SRF=subretinal fluid;RPE=retinal pigment epithelium.
*Treatment (time, weeks); **BCVA before intravitreal bevacizumab; †follow-up duration after intravitreal bevacizumab injection; ‡Localized bleeding at 6 weeks after PDT plus intravitreal bevacizumab.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download