Abstract
Methods
In one patient, the refraction of both eyes (the left eye was injured, but the right eye was not) was measured with an autorefractometer. The cycloplegic refraction was measured at the early stage of trauma and again 3 months after the blunt eye injury. The angle and depth of the anterior chamber, the ciliary body, and the choroids were examined by ultrasound biomicroscopy (UBM) over 3 months. The depth of the anterior chamber, the thickness of the lens, and the axial length were measured by A-scan ultrasonography in both eyes. During the 3 months after the injury, we made comparisons between the menifest and the cycloplegic refractions, the depths of anterior chambers, the thickness of the lenses, the axial lengths, and the UBM-determined appearances of the angles and depths of the anterior chambers, the ciliary bodies, and the choroids in both eyes.
In 1870, Kugel published the first paper on myopia following a trauma. Since then, transient myopias after traumas often have been reported, however we currently refer to the condition as myopia after a blunt eye trauma. Many authors have reported on myopic changes following traumas and these cases were almost always transient. In these cases, the myopic changes were -1 to -6 diopters and the refractive changes were recovered and normalized in nearly all patients within a month.1-4,6
The cause of myopia after trauma has been attributed to many different mechanisms. Duke1 reported that ciliary spasm and attenuation of the ciliary zonule caused myopias in patients who had blunt traumas. Doton and Oliver2 reported that myopia after a blunt trauma was due to a uveal effusion and a shallow anterior chamber. In contrast, Kutner3 reported that the occurrence of myopic change and acute angle closure glaucoma was due to anterior shifting of the lens-iris plane. While Steele et al.4 reported that the myopic change was -4.75 to -5.25 diopters with an A-scan and the myopic change was due to an increase in the anterior to posterior diameter of the lens. Moreover, the anterior to posterior diameter of the lens increased because of edema in the ciliary body without anterior shifting of the lens-iris plane. On the other hand, Romem et al.5 mentioned that transient myopia was -1.5 to -4.0 diopters and reported that transient glaucoma could occur through a similar mechanism. Ikeda et al.6 found that the uveoscleral outflow increased with UBM and reported that this event could bring about myopic change through anterior shifting of the lens-iris plane and thus decreasing the depth of the anterior chamber. In general, myopia following a blunt trauma is almost always transient with the reported refractive changes ranging from -1 diopter to -6 diopters. Most of the myopic changes are reported to normalize within a month.1-4,6
We have observed a patient with a myopic change of -6 diopters due to spasm and edema of the ciliary body following a blunt trauma to the left eye and have followed this patient's recovery for 3 months. Until now, no domestic report has been made on the pathogenesis of myopic changes following blunt trauma. Therefore we report on this case to study the etiology of myopic change using ultrasonography and other various examinations.
A young female patient visited the department of ophthalmology in our hospital with a chief complaint of decreased visual acuity 4 hours following a blunt trauma to the eye. We checked the visual acuity without correction as well as the corrected visual acuity and then performed manifest refraction, cycloplegic refraction, and A-scan ultrasonography.
Ultrasound biomicroscopy (UBM) examination was performed 4 times on each section that was divided by a 90 degree interval in a normal eye, and 12 times on each section that was divided by a 30 degree interval in a traumatized eye (UBM Model 480, Humphrey Instrument Ind., San Leandro, CA) at the 4 week time point and the 3 month time point after the trauma. The thickness of the ciliary body was measured with an imaginary line that was vertical to the corneal endothelium and passed through the scleral spur (black arrows in Fig. 1, 2, 3). Refractive power was measured with a TOPCON KR-8100 Autorefractor and the cycloplegic refraction was performed under the maximal dilated state after dropping a cycloplegic agent (Cyclopentolate). All measurements were repeated 3 times and the mean values are reported. Examination for the anterior chamber angle was performed with a Goldmann four mirror lens to find whether cyclodialysis exists or not. After cycloplegic refraction, ultrasound examination was performed 7 times with the Humphrey A-scan system 835. We took an average of 5 values that excluded the maximal and minimal values. We also measured the anterior to posterior diameter of the lens, the depth of the anterior chamber, and the axial length. The units for these values are reported in millimeters. Intraocular pressure was measured 3 times with a non-contact tonometer, TOPCON CT-80 and reported as a mean value.
The patient was a 16-year-old female, who was beaten with clenched fists by other adlescents 4 hours before her visit to our hospital. This patient presented with a periorbital contusion, rib fractures, and so on. The naked visual acuity was 1.0 in the right eye and 0.15 in the left eye. The intraocular pressure was 11 mmHg in the right eye and 15 mmHg in the left eye. We found that the intraocular pressure in the left eye was relatively high within a normal range. We could not perform accurate refraction because of the severely painful periorbital swelling of the left eye at the time of her admission. In a slit-lamp examination, we found left corneal epithelial defects and an anterior chamber reaction with inflammatory cells (+1) without hyphema. The peripheral retina was edematous, but the macula was normal when the patient was examined by a fundus examination. The B-scan revealed no specific finding in both eyes.
A few months before the trauma, the patient's naked visual acuities had been 1.0 in both eyes on a visual acuity test that had been performed at her school. Furthermore, she had never experienced discomfort due to the difference between her bilateral visual acuities.
Examinations including refraction were performed as the periorbital swelling decreased. The naked visual acuities were 1.0 in the right eye and 0.3 in the left eye. The right and left eye intraocular pressures were similar, with the right eye measuring 12 mmHg and the left eye measuring 11 mmHg. The manifest refraction was +0.25 D (D: diopter) sph=-0.25 D cyl ×160°A in the right eye and -6.00 D sph=-0.50 D cyl ×150°A in the left eye. The corrected visual acuity of the left eye was 0.8. The cycloplegic refraction was +0.25 D sph= -0.25 D cyl ×160°A in the right eye and -3.75 D sph= -0.25 D cyl ×160°A in the left eye. The fundus examination revealed that the retinal edema was decreased. Our findings for the gonioscopic examination were normal without cyclodialysis. When measured by ultrasonography, we found that the depths of the anterior chamber were 3.48 mm in the right eye, 3.28 mm in the left eye, and the depth of a traumatic eye (left) was shallower than the depth of the non-traumatized eye (right) by approximately 0.2 mm. The thicknesses of both lenses were 3.43 mm in the right eye, 3.82 mm in the left eye, which was 0.39 mm or thicker in the traumatized eye. The axial lengths were similar with 23.92 mm in the right eye and 23.87 mm in the left eye.
In the left eye, the naked visual acuity was 0.4, the intraocular pressure was 13 mmHg, and the refraction result was -4.25 D sph= -0.50 D cyl ×160°A. In addition, the corrected visual acuity was 0.8. In the cycloplegic refraction of the left eye, the result was -3.25 D sph= -0.25 D cyl ×160°A. The difference between the results of the menifest and cycloplegic refractions got smaller. When we performed a fundus examination of the left eye, we found that retinal edemas had disappeared and were otherwise normal. An ultrasonographic examination of the left eye revealed that the depth of the anterior chamber was 3.34 mm, the thickness of the lens was 3.74 mm, and the axial length was 23.85 mm.
One month after the trauma, we examined the left eye and found that the naked visual acuity was 0.5, the intraocular pressure was 13 mmHg, and the refraction result was -3.00 D sph = -0.50 D cyl ×170°A. The corrected visual acuity with correction was 0.9. When we performed a cycloplegic refraction of the left eye, the result was -2.75 D sph= -0.50 D cyl ×160°A.
An ultrasonographic examination of the left eye revealed that the depth of anterior chamber was 3.39 mm, the thickness of the lens was 3.63 mm, and the axial length was 23.92 mm. Ultrasonographic examinations of anterior segments in both eyes were performed due to continuous myopia, and we evaluated the differences. Though both anterior chamber angles were open, when we compared the thickness of the left ciliary body of about 1.73 mm with the 1.47 mm thickness in the right eye, we found edema and diffuse thickening of the ciliary body (white arrow) in the left eye (Fig. 1, 2).
In the left eye, the naked visual acuity was 0.7, the intraocular pressure was 13 mmHg, and the result of refraction was -1.75 D sph= -0.25 D cyl ×180°A. The corrected visual acuity was 1.0. The result of cycloplegic refraction of the left eye was -1.50 D sph= -0.75 D cyl ×170°A. In an ultrasonographic examination of the left eye, the depth of the anterior chamber was 3.44 mm, the thickness of the lens was 3.51 mm, and the axial length was 23.88 mm.
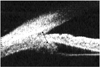
Three months after the trauma, the naked visual acuity of the left eye was 0.8, the intraocular pressure was 12 mmHg, and the refraction result was -0.25 D sph= -0.25 D cyl ×170°A. In addition, the corrected visual acuity was 1.0. The result of cycloplegic refraction of the left eye was -0.25 D sph= -0.25 D cyl ×170°A. The ultrasonographic examination of the left eye showed that the depth of anterior chamber was 3.46 mm, the thickness of the lens was 3.49 mm, and the axial length was 23.89 mm. Finally, an Ultrasonographic examination of the anterior segment of the left eye showed that the degree of edema of the ciliary body was diminished to 1.40 mm (Fig. 3).
Myopic changes following a blunt trauma have been reported by a number of authors. In these reports, the changes were transient in most case, ranging from -1 to -6 diopters. Moreover, the myopic changes were diminished and normalized within approximately one month.1-4,6
In our case, we report that the traumatized eye showed a myopia of -6.00 diopters immediately after the trauma and that the myopia had recovered to -0.25 diopters 3 months after the trauma. Ciliary spasm and attenuation of the ciliary zonule1 have previously been mentioned as the causes of the change in the refractive power.
We studied the menifest and cycloplegic refractive powers in our patient for the three months during recovery. We found that the difference between the menifest and cycloplegic refractive power changed during this period. Indeed, we found that the difference between menifest and cycloplegic refractive power was about 2.25 diopters at the first examination upon admission, and this was reduced to 0.25 diopters when the patient was examined one month after the trauma. This difference in refractive power decreased even more until it was finally the same in the latter two months of the 3-month study period. As the myopia was reduced with a cycloplegic agent, we thought that ciliary spasm was one cause of the myopic change. Our data shows that ciliary spasms almost disappeared and thus were normalized within one month (Table 1).
The reported causes of myopic change include a change in the axial length, anterior shifting of the lens-iris plane, and decrease in the depth of the anterior chamber. One report found that all of these causes induced an occurrence of acute angle closure glaucoma.3 Another report found that myopic change could occur due to anterior shifting of the lens-iris plane and decrease the depth of the anterior chamber.6 In fact, we found that the depth of the anterior chamber in a traumatized eye right after a trauma was 3.28 mm, which was shallower than the anterior chamber of a non-traumatized eye by 0.2 mm. Furthermore, the depth of the anterior chamber in a traumatized eye increased by a depth of 3.46 mm, which was similar to the depth in the opposed eye.
Thus anterior shifting of the lens-iris plane and a decrease in the depth of the anterior chamber appears to influence the occurrence of myopia, though the influence of these factors was tiny (Table 2). However, we could exclude that the myopic change was not due to the difference between both axial lengths because the difference between the axial length of the traumatized and non-traumatized eyes was not so large (Table 2). Moreover, we found no glaucomatic manifestation related to the decrease in the depth of the anterior chamber because the intraocular pressure was within a normal range.
Previous reports have found that the increase in anterior to posterior diameter of the lens led to a myopic change. Additional work found that the ciliary edema without anterior shifting of the lens-iris plane4 is related to the increase in anterior to posterior diameter of the lens. In our case, the thickness of the lens in the traumatized eye was 3.82 mm immediately after the trauma, which was thicker than the lens of the non-traumatized eye by 0.41 mm. The thickness became thinner over a 3-month period with a final measurement of 3.49 mm, which was as thin as the lens of the eye without trauma (Table 2). In a UBM examination one month after the trauma when the ciliary spasm had almost disappeared, we found edema of the ciliary body in the traumatized eye. Ultrasonographic examination of the anterior segment of the left eye three months following the trauma found that the degree of edema of the ciliary body was diminishing. This implies that the change in the lens thickness as well as the edema of the ciliary body can indicate myopic change following a trauma (Fig. 1, 2, 3).
A few drugs can cause transient myopia without uveal effusion.7 In these cases, a hypersensitivity reaction is thought to trigger the edema of the ciliary body.8 The ciliary edema following a trauma could be caused by the synaptic ends of sympathetic nervous system that are present on the vascular walls in the ciliary body. Thus, an increase in the vascular permeability related to the sympathetic paralysis following a trauma could be related to the ciliary edema.9
Finally, unstable changes in intraocular pressures such as low intraocular pressure with cyclodialysis and a transient increase in intraocular pressure after the closure of a ciliary dissociation frequently follow most blunt eye traumas.10-15 In our case, intraocular pressure was found to be within a normal range, with no hyphema. In putting these results together, the mechanism of changes in the refractive power after a trauma includes the ciliary spasm, a decrease in the depth of the anterior chamber caused by the anterior shifting of the lens-iris plane, an increase in the anterior to posterior lens diameter, and ciliary edema. Among those mechanisms, considering an acute decrease in the difference between manifest refractive power and cycloplegic refractive powers for a 1-monthd period, we knowledge that myopia due to the ciliary spasm continues for one month or so and that myopia by other mechanisms continues through a 3-3-month period with a slow recovery.
In conclusion, the change in refractive power after a trauma is related to the ciliary spasm, a decrease in the depth of the anterior chamber caused by the anterior shifting of the lens-iris plane, an increase in the anterior to posterior lens diameter, and ciliary edema. Thus, UBM of the anterior segment and ultrasonographic examination of the traumatized eye are helpful for the diagnosis and confirmation of those changes.
Figures and Tables
Fig. 1
Ultrasound microscopy on the right eye shows a normal chamber angle and the ciliary body at 1 month post-trauma. Note that there was no thickness of the ciliary body
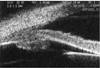
Fig. 2
Ultrasound microscopy on the left eye shows the swelling of the ciliary body at 1 month post-trauma. The chamber angle shows normal structure and diffuse thickening of the ciliary body.
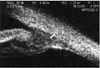
Fig. 3
Ultrasound microscopy of left eye shows decrement and swelling of the ciliary body at 3 months post-trauma. Note the resolution of the diffuse thickening of the ciliary body.
References
1. Duke Elder S. System of Ophthalmology. 1970. Vol. 5. St.Louis: Mosby;354–355.
2. Dotan S, Oliver M. Shallow anterior chamber and uveal effusion after nonperforating trauma to the eye. Am J Ophthalmol. 1982. 94:782–784.
3. Kutner BN. Acute angle closure glaucoma in nonperforating blunt trauma. Arch Ophthalmol. 1988. 106:19–20.
4. Steele CA, Tullo AB, Marsh IB, Storey JK. Traumatic myopia; an ultrasonographic and clinical study. Br J Ophthalmol. 1987. 71:301–303.
5. Romem M, Isakow I, Dolev Z. Posttraumatic transient glaucoma and myopia. Am J Ophthalmol. 1985. 99:495.
6. Ikeda N, Ikeda T, Nagata M, Miura O. Pathogenesis of transient high myopia after blunt trauma. Ophthalmology. 2002. 109:501–507.
7. Galin MA, Baras I, Zweifach P. Diamox induced myopia. Am J Ophthalmol. 1962. 54:237–240.
8. Howes EL Jr, Mckay DG. Circulating immune complexes. Effects on ocular vascular permeability in the rabbit. Arch Ophthalmol. 1975. 93:365–370.
9. Fleming DG, Hall JL. Autonomic innervation to ciliary body. Am J Ophthalmol. 1959. 48:287–293.
10. Shea M, Mednick EB. Ciliary body reattachment in ocular hypotony. Arch Ophthalmol. 1981. 99:278–281.
11. Barasch K, Galin MA, Baras I. Postcyclodialysis hypotony. Am J Ophthalmol. 1969. 68:644–645.
12. Chandler PA, Maumenee AE. Major cause of hypotony. Trans Am Acad Ophthalmol Otolaryngol. 1961. 65:563–575.
13. Shaffer RN, Weiss DI. Concerning cyclodialysis and hypotony. Arch Ophthalmol. 1962. 68:25–31.
14. Brubaker RF, Pederson JE. Ciliochoroidal detachment. Surv Ophthalmol. 1983. 27:281–289.
15. Kuchle M, Naumann GO. Direct cyclopexy for traumatic cyclodialysis with persisting hypotony. Ophthalmology. 1995. 102:322–323.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download