Abstract
Purpose
To report two cases of femtosecond laser-assisted small incision deep lamellar endothelial keratoplasty (DLEK) for patients with corneal endothelial decompensation by Fuchs dystrophy and glaucoma
Methods
Femtosecond laser (IntraLase®; IntraLase Corp., Irvine, CA) with 15 kHz of repetition rate, was used for a 9.5 mm diameter by 400 µm thickness donor corneal lamellar dissection.
Results
In Case 1, the graft was clear and compact without interface haze, Orbscan showed smooth and regular corneal surface, specular microscopy was unremarkable without sign of corneal endothelial damage, and Optical coherence tomography showed uniform graft well attached to recipient stroma with minimal interface reflection at 2 months postoperation. In Case 2, the graft was clear and compact with minimal interface haze at 1 month postoperation. Femtosecond laser-assisted small incision DLEK was safe and technically feasible in our cases; however, further evaluation is required to determine long-term effects.
Gerrit Melles first introduced the 'Posterior lamellar keratoplasty' technique in 1998.1,2 Mark Terry and Paula Ousley modified this technique to 'Deep lamellar endothelial keratoplasty' (DLEK).3,4 Previously, the original large incision technique was modified by both Melles and Terry to a small incision technique.5,6 The small incision technique has theoretical advantages, which may reduce corneal flattening and refractive astigmatism, thereby resulting in better visual outcome.5 However, manual lamellar dissection is time consuming and technically difficult, and may result in corneal perforation and interface scarring.6-8
Recent progress in refractive surgery has led to femtosecond (10-15 seconds) laser use in performing lamellar keratoplasty. The laser uses an infrared wavelength (1053 nm) scanning pulse, focused to 3 µm with an accuracy of 1 µm, to create precise lamellar cornea dissections.9 According to recent papers, femtosecond laser use demonstrated improved flap uniformity and better flap thickness predictability than mechanical microkeratomes.9-12
In this report, we describe 'Femtosecond laser-assisted small incision DLEK' for treating patients with corneal endothelial dysfunction. By applying the femtosecond laser, we may be able to perform lamellar keratoplasty more easily and rapidly while reducing corneal perforation and interface scarring risk.
Topical anesthesia was used for the surgery. The donor corneoscleral button was mounted in a dedicated artificial anterior chamber (Bausch & Lomb, St. Louis, MO). Ultrasound pachymetry was performed prior to donor lamellar dissection. This was to assure a donor corneal thickness between 500 and 565 µm, in order to approximate a graft thickness of 100 to 165 µm. The femtosecond laser (IntraLase®; IntraLase Corp., Irvine, CA) was then used to create a corneal flap of 9.5 mm diameter and 400 µm thickness. Femtosecond laser parameter settings were as follows: raster energy 3.6 µJ, spot separation 10 µm, line separation 10 µm, side cut energy 3.8 µJ, spot size 2.4 µm, and repetition rate 15 kHz. Detailed specifications are shown in Table 1. After laser application, stromal and side cut adhesions were fully released and the anterior corneal flap was fully lifted. The donor corneoscleral button was then placed in medium and transported to the operating room, where the anterior flap was excised with scissors and an 8.0 mm trephine (Katena Products, Inc., Denville, NJ) was used to punch out the posterior donor corneoscleral button.
The recipient corneal lamellar dissection and graft transplantation was performed as previously described.5,6 Briefly, following an 8.0 mm epithelial marking and 5.0 mm scleral incision, a deep lamellar pocket was created to approximately 75-85% of corneal thickness. The posterior recipient disc was then excised with Cindy scissors (Bausch & Lomb) using the 8.0 mm epithelial mark as a template. The excised posterior recipient disc was removed from the lamellar pocket and spread over recipient corneal epithelium to verify size. An 8.0 mm pre-prepared posterior donor disc was then folded, endothelial side inside, with a layer of viscoelastic (Healon®; Pharmacia, Peapack, NJ) coating the endothelium and inserted into the anterior chamber through the small incision using forceps. The folded donor disc was opened and attached to the recipient stromal bed by injecting air underneath the graft. The scleral wound was closed with 2 or 3 interrupted 10-0 nylon sutures and air was replaced with balanced salt solution to normalize intraocular pressure (IOP).
Femtosecond laser-assisted small incision DLEK was performed in the right eye of a 73-year-old male with Fuchs dystrophy (Fig. 1). Preoperative uncorrected visual acuity (UCVA) and best spectacle corrected visual acuity (BSCVA) was 20/100 and 20/80 respectively and manifest refraction was +0.75 -1.75×30. Table 2 summarizes the clinical data. Donor corneoscleral button lamellar dissection was performed using IntraLase femtosecond laser (IntraLase Corp.), set at a depth of 400 µm and flap diameter of 9.5 mm. Surgery was performed as described above.
At two months postoperation, the graft was clear and compact without interface haze (Fig. 2A). UCVA and BSCVA was 20/150 and 20/100 respectively and manifest refraction was +2.75 -1.00×50. Corneal topography showed smooth and regular corneal surface and specular microscopy was unremarkable without sign of corneal endothelial damage (Fig. 2B and C). Optical coherence tomography (OCT) findings showed compact and uniform graft, well attached to the recipient stromal bed with minimal interface reflection (Fig. 3).
Femtosecond laser-assisted small incision DLEK was performed in the left eye of a 94-year-old female with corneal edema, glaucoma, and dry type age-related macular degeneration (ARMD). Her preoperative UCVA and BSCVA was 20/400 and 20/200 respectively and her manifest refraction was -1.00 -1.75×95. IOP was well controlled with topical medication (Cosopt®; Merck & Co., Whitehouse Station, NJ). Table 2 summarizes clinical data. Surgery was uneventfully performed as described above. At one week postoperation, the graft was clear and compact, IOP was 19 mmHg, and UCVA was 20/400. At one month, the graft was clear and compact with minimal interface haze; IOP measured 31 mmHg. Anti-glaucoma medication (Alphagan®; Allergan, Irvine, CA) was added and topical steroid tapered. We were not able to perform further examinations due to follow-up loss at two months postoperation.
All participants provided informed consent before participating. All methods adhered to the Declaration of Helsinki of the World Medical Association for research involving human subjects.
Posterior keratoplasty refers to removal of diseased posterior corneal layers (endothelium, Descemet's membrane, and posterior stroma) and replacement by partial-thickness donor tissue. This procedure can be an attractive alternative to penetrating keratoplasty (PKP) in treating corneal endothelial diseases such as Fuchs dystrophy and aphakic and pseudophakic bullous keratopathy.13-15 The endothelial replacement technique via scleral incision was first described by Melles and later modified by Terry and Ousley to 'Deep lamellar endothelial keratoplasty' (DLEK).3,4 Recent modification includes introducing the folded donor disc through the a small incision.6
One problem in performing DLEK is the technical difficulty associated with manual lamellar dissection. It is not only time consuming, but also has the potential risk of corneal perforation.8 Moreover, even with uncomplicated dissection, the plane may not be smooth and uniform, which results in interface scarring and reduction in potential best vision.6,7
In a recent paper, Azar and coauthors described microkeratome-assisted posterior keratoplasty.16 Given that Laser-Assisted in situ Keratomileusis (LASIK) shows almost no interface haze on the cornea,17,18 this technique's potential advantage is less interface scarring than manual dissection. However, microkeratome-assisted lamellar dissection does have the risk of corneal perforation in deeper dissection.
Recently, the femtosecond laser was introduced in refractive surgery fields for LASIK flap creation. According to recent papers, it demonstrated improved flap uniformity and better flap thickness predictability than mechanical microkeratomes.9-12,19
Similar to the microkeratome, femtosecond laser technology has evolved. After initial procedures with a 2 kHz laser in 1996, 15 kHz (2003), 30 kHz (2005), and soon after, 60 kHz (2006) engines were introduced.
The femtosecond laser creates corneal resection by delivering laser pulses tissue microphotodisruption. Pulses are scanned and placed in a vertical pattern for trephination (side) cuts or in a spiral or raster (zigzag) pattern to achieve lamellar cuts. The smoothness of optical surfaces is determined by programmable parameters: energy per pulse, separation of adjacent laser spots (spot separation), and raster pattern row spacing (line separation). The closer the laser spots, the less energy required.20 The IntraLase system scans tissue at a repetition rate which depends on laser engine capacity. Higher repetition rates allow use of lower pulse energy and closer spot/line separation settings, which results in a smoother lamellar interface, an easier anterior corneal flap lift, and a faster procedure. The newer 60 kHz engine permits a line/spot separation down to 6×6 µm and energy less than 1 µJ per pulse, which may show superior efficiency for harvesting the posterior corneal disk.21
One concern during preoperative femtosecond laser-assisted donor eye posterior lamellar discs (PLDs) preparation is the possibility of endothelial cell loss (ECL). ECL has been reported at 4% after 150 to 200 µm thick endothelial side PLDs preparations, and did not appear to be caused by laser pulse energy.22 Another in vitro study reported 4.3% versus 7.7% ECL after 30 kHz laser lamellar cutting compared to 15 kHz laser for horizontal lamellar cuts at corneal depth of 400 µm and 9.5 mm diameter.23 Favorable outcomes are consistent with histological results showing adjacent thermal damage to be on the order of 1 µm.24 Clinical results from Case 1 showed relatively intact postoperative endothelium status, which was comparable to previous reports. The recently released 60 kHz engine could minimize ECL risk by reducing delivered energy.
Moreover, the femtosecond laser is expected to be applied in full-thickness PKP. Cuts are customizable to achieve different graft geometric configurations, potentially allowing for sutureless, self-adhesive, or shaped keratoplasty. Two or more side/lamellar cut segments can be combined to create patterns for shaped keratoplasty, including top-hat (larger diameter cut posteriorly), mushroom (larger diameter cut anteriorly), zigzag, and Christmas tree patterns.25 Peripheral wound edge shaping can provide stronger healing by increasing surface area, reduce corneal astigmatism by decreasing suture number, reduce donor-host topographic distortion disparity, and quicken visual recovery.
Following we describe small incision DLEK by femtosecond laser for donor corneal lamellar dissection.
Before performing femtosecond laser-assisted donor lamellar dissection, we measured donor corneal thickness by ultrasound pachymetry. If corneal thickness was thicker than 565 µm, glycerin was applied to dehydrate the cornea, and avoid a thinner graft than intended, as well as to ascertain graft thickness of 100 to 165 µm for transplantation. The laser was then used to create a corneal flap of 9.5 mm diameter and 400 µm thickness. Afterwards, two possible methods to obtain the posterior donor disc were used. In the first method, stromal adhesions are released without fully lifting the flap. This bi-hinged donor corneoscleral button is then placed in medium, transported to the operating room, and punched out with an 8.0 mm trephine. Separation of the anterior and posterior donor corneal disc follows. In the second method, stromal and side cut adhesions are fully released, and the anterior corneal flap is fully lifted. This donor corneoscleral button is placed in medium and transported; following, the anterior flap is excised using scissors and the posterior part is punched out with an 8.0 mm trephine. In our cases we used the second method for posterior donor disc preparation. Although the disadvantage is using scissors for anterior cap removal, there are other advantages. By removing the anterior cap, it is easier to visualize the posterior donor button edge, which may reduce a decentered donor cut. Furthermore, anterior and posterior donor disc separation is much easier, since the anterior flap is fully lifted and removed before trephination.
Case 1 clinical results showed a clear and compact graft without interface haze at two months postoperation. These findings were further confirmed in OCT, which showed a clear and uniform graft with minimal interface reflection and good graft attachment. Despite good clinical findings, postop visual acuity was not as expected. However, the patient was satisfied and his vision fluctuation (previously worse in the morning) was eliminated. Considering findings and short-term follow-up, we may expect further improvement with time. Case 2 clinical results also showed clear and compact graft with minimal interface haze. However, postop visual acuity did not improve because of low visual potential from dry ARMD and glaucoma. Unfortunately, this patient had an IOP increase at one month postoperation and was unable to follow-up. This increase may be due to either frequent steroid use or glaucoma progression.
We can assume several possible factors for the unexpected postoperative visual acuity. Visual acuity after either PKP or DLEK is mainly a function of retinal macular potential and cornea optical quality. In studies of PKP in young patients with keratoconus, postoperative visual results are uniformly better compared to results in older Fuchs' dystrophy patients, despite similar graft problems of high or irregular astigmatism.26 This suggests that older maculas, may play a role in visual loss after any form of corneal transplantation, including DLEK.
In contrast to PKP where the cornea contributes to refractive visual loss, often resulting from high or irregular astigmatism, in DLEK, topography is usually normal and corneal visual loss is most likely attributed to the donor-recipient stromal interface. The interface in DLEK may account, on average, for 1 line of visual loss compared to the individual macular potential vision.27 Other potential corneal factors include increased corneal thickness and posterior corneal curvature. We observed irregular posterior floating map corneal curvature in Case 1's postoperative topography. How this irregularity influences visual acuity requires further investigation.
Due to lack of long term follow-up and small case number, we can not fully determine potential advantages of 'femtosecond laser-assisted small incision DLEK'. However, these cases demonstrate graft uniformity, technical feasibility, and potential application of femtosecond laser in lamellar keratoplasty. Further evaluation with longer follow-up and more cases would reveal potential advantages. Unfortunately, the use of femtosecond laser in recipient corneas is currently limited by the Institutional Review Board. Also the femtosecond laser is not approved for cuts deeper than 400 µm; therefore recipient cornea posterior lip creation requires scissors. Hopefully in the near future, we can use the femtosecond laser and perform both donor and recipient corneal lamellar dissection.
Figures and Tables
 | Fig. 1Preoperative photograph of a 73-year-old male showing corneal edema and guttata with beaten metal appearance (A). Specular microscopy shows coalesced guttata excrescences in the form of dark structures (cell density 1363 cell/mm2, coefficient of variation 0.44, hexagonality 54%) (B). |
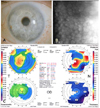 | Fig. 2Postoperative photograph of a 73-year-old male two months after femtosecond laser-assisted small incision DLEK surgery showing clear and compact graft without interface haze (A, Top left). Specular microscopy shows no endothelial cell damage after surgery (cell density 1962 cell/mm2, coefficient of variation 0.39, hexagonality 56%) (B, Top right). Orbscan shows smooth and regular corneal curvature (C, Bottom). |
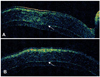 | Fig. 3Optical coherence tomography (OCT) of a 73-year-old male two months after femtosecond laser-assisted small incision DLEK surgery. Vertical (A) and horizontal (B) OCT of the cornea show compact and uniform graft which is well attached to recipient stroma with minimal interface reflection (Arrowheads). |
References
1. Melles GR, Eggink FA, Lander F, et al. A surgical technique for posterior lamellar keratoplasty. Cornea. 1998. 17:618–626.
2. Melles GR, Lander F, Beekhuis WH, et al. Posterior lamellar keratoplasty for a case of pseudophakic bullous keratopathy. Am J Ophthalmol. 1999. 127:340–341.
3. Terry MA, Ousley PJ. Endothelial replacement without surface corneal incisions or sutures: topography of the deep lamellar endothelial keratoplasty procedure. Cornea. 2001. 20:14–18.
4. Terry MA, Ousley PJ. Deep lamellar endothelial keratoplasty in the first United States patients: early clinical results. Cornea. 2001. 20:239–243.
5. Melles GR, Lander F, Nieuwendaal C. Sutureless, posterior lamellar keratoplasty: a case report of a modified technique. Cornea. 2002. 21:325–327.
6. Terry MA, Ousley PJ. Small incision deep lamellar endothelial keratoplasty (DLEK): six-month results in the first prospective clinical study. Cornea. 2005. 24:59–65.
7. Soong HK, Katz DG, Farjo AA, et al. Central lamellar keratoplasty for optical indications. Cornea. 1999. 18:249–256.
8. Chau GK, Dilly SA, Sheard CE, Rostron CK. Deep lamellar keratoplasty on air with lyophilised tissue. Br J Ophthalmol. 1992. 76:646–650.
9. Sugar A. Ultrafast (femtosecond) laser refractive surgery. Curr Opin Ophthalmol. 2002. 13:246–249.
10. Nordan LT, Slade SG, Baker RN, et al. Femtosecond laser flap creation for laser in situ keratomileusis: six-month follow-up of initial U.S. clinical series. J Refract Surg. 2003. 19:8–14.
11. Binder PS. Flap dimensions created with the IntraLase FS laser. J Cataract Refract Surg. 2004. 30:26–32.
12. Kezirian GM, Stonecipher KG. Comparison of the IntraLase femtosecond laser and mechanical keratomes for laser in situ keratomileusis. J Cataract Refract Surg. 2004. 30:804–811.
13. Ing JJ, Ing HH, Nelson LR, et al. Ten-year postoperative results of penetrating keratoplasty. Ophthalmology. 1998. 105:1855–1865.
14. Melles GR, Remeijer L, Geerards AJ, Beekhuis WH. The future of lamellar keratoplasty. Curr Opin Ophthalmol. 1999. 10:253–259.
15. Chung SE, Lee DH, Chung TY, Chung ES. Results of Deep Lamellar Endothelial Keratoplasty (DLEK). J Korean Ophthalmol Soc. 2006. 47:1743–1750.
16. Azar DT, Jain S. Microkeratome-assisted posterior keratoplasty. J Cataract Refract Surg. 2002. 28:732–733.
17. Wachtlin J, Langenbeck K, Schrunder S, et al. Immunohistology of corneal wound healing after photorefractive keratectomy and laser in situ keratomileusis. J Refract Surg. 1999. 15:451–458.
18. Jain S, Khoury JM, Chamon W, Azar DT. Corneal light scattering after laser in situ keratomileusis and photorefractive keratectomy. Am J Ophthalmol. 1995. 120:532–534.
19. Kim SA, Choi ES, Lee TH, et al. Comparision of Corneal Endothelial Change in Femtosecond Laser and Microkeratome LASIK. J Korean Ophthalmol Soc. 2005. 46:2059–2064.
20. Sarayba MA, Ignacio TS, Binder PS, Tran DB. Comparative study of stromal bed quality by using mechanical, IntraLase femtosecond laser 15-and 30 kHz microkeratomes. Cornea. 2007. 26:446–451.
21. Sarayba MA, Ignacio TS, Tran DB, Binder PS. A 60 kHz IntraLase femtosecond laser creates a smoother LASIK stromal bed surface compared to a Zyoptix XP mechanical microkeratome in human donor eyes. J Refract Surg. 2007. 23:331–337.
22. Sarayba MA, Juhasz T, Chuck RS, et al. Femtosecond laser posterior lamellar keratoplasty: a laboratory model. Cornea. 2005. 24:328–333.
23. Cheng YY, Pels E, Cleutjens JP, et al. Corneal Endothelial Viability After Femtosecond Laser Preparation of Posterior Lamellar Discs for Descemet-Stripping Endothelial Keratoplasty. Cornea. 2007. 26:1118–1122.
24. Jonas JB, Vossmerbaeumer U. Femtosecond laser penetrating keratoplasty with conical incisions and positional spikes. J Refract Surg. 2004. 20:397.
25. Mian SI, Shtein RM. Femtosecond laser-assisted corneal surgery. Curr Opin Ophthalmol. 2007. 18:295–299.
26. Terry MA, Ousley PJ. Deep lamellar endothelial keratoplasty visual acuity, astigmatism, and endothelial survival in a large prospective series. Ophthalmology. 2005. 112:1541–1548.
27. Terry MA, Ousley PJ. Rapid visual rehabilitation after endothelial transplants with deep lamellar endothelial keratoplasty (DLEK). Cornea. 2004. 23:143–153.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download