Abstract
We report four cases in which a pericardium (Tutoplast®) plug was used to repair a corneoscleral fistula after Ahmed Glaucoma Valve (AGV) explantation. In four cases in which the AGV tube had been exposed, AGV explantation was performed using a pericardium (Tutoplast®) plug to seal the defect previously occupied by the tube. After debridement of the fistula, a piece of processed pericardium (Tutoplast®), measured 1 mm in width, was plugged into the fistula and secured with two interrupted 10-0 nylon sutures. To control intraocular pressure, a new AGV was implanted elsewhere in case 1, phaco-trabeculectomy was performed concurrently in case 2, cyclophotocoagulation was performed postoperatively in case 3 and anti-glaucomatous medication was added in case 4. No complication related to the fistula developed at the latest follow-up (range: 12~26 months). The pericardium (Tutoplast®) plug seems to be an effective method in the repair of corneoscleral fistulas resulting from explantation of glaucoma drainage implants.
Glaucoma drainage implants (GDIs) have been an effective therapeutic option in the management of refractory glaucoma. The complications associated with GDIs, however, include hypotony, choroidal effusion, corneal decompensation, cataract, endophthalmitis, diplopia, and migration of the implant.1,2 Erosion of the silicone tube through the overlying conjunctiva has also been recognized, and various methods such as a conjunctival advancement, a conjunctival patch graft, an amniotic membrane patch graft or an interpolated conjunctival pedicle flap have been described to cover the exposed tube.3-6 However, if these methods turn out unsuccessful or can not be utilized and the patient has implant-related intractable pain, inflammation, or endophthalmitis, the GDIs may have to be explanted from the eye.
Explantation of a GDIs necessitates the repair of the fistula in the cornea and the sclera, which was previously occupied by the silicone tube. The corneoscleral fistula, if repaired with sutures alone, may lead to significant astigmatism or wound dehiscence. Sibayan et al.7 first reported the successful use of a processed pericardium to manage the corneoscleral fistula after explantation of GDIs. We describe four cases in which a pericardium (Tutoplast®, Innovative Ophthalmic Products, Costa Mesa, California, USA) plug was used to repair a corneoscleral fistula after Ahmed glaucoma valve (AGV New World Medical, Inc, Rancho Cucamonga, California, USA) explantation, and the postoperative outcomes from these cases which have been followed up for 12 months or longer.
Case 1: A 49-year-old man with neovascular glaucoma (NVG) in the right eye (RE), who had undergone phacoemulsification, posterior chamber lens (PCL) implantation, and AGV implantation, was referred to us. His intraocular pressure (IOP) (RE) was 40 mmHg despite medical therapy including dorzolamide 2% / timolol maleate 0.5% fixed combination (Cosopt®, Merck & Co., Inc., Blue Bell, PA, USA), latanoprost 0.005% (Xalatan®, Pfizer, Inc., New York, NY, USA), and brimonidine purite 0.15% (Alphagan P® 0.15%; Allergan, Inc., Irvine, CA, USA). The AGV tube as well as its body was found exposed with complete melting of the patch graft and conjunctiva. The body of the valve was located close to the limbus (4 mm posterior to the limbus): in addition, this diabetic patient suffered from severe ocular pain (Fig. 1). Surgery was planned to remove the preexisting AGV and insert a new AGV in another quadrant because we believed that the preexisting AGV was non-functioning and pain-evoking. In addition, we believed that insertion of a new AGV into the same place might predispose the patient to a recurrence of exposure.
Under peribulbar anesthesia, a conjunctival peritomy was performed alongside the area of transconjunctival tube erosion, and the tube of the AGV was removed from the anterior chamber. To seal the space previously occupied by the tube, the fistula was debrided with a No. 11 Bard-Parker blade and a Weckcel® sponge. A piece of processed pericardium (Tutoplast®), measured 1 mm in width, was then plugged into the fistula. The pericardium was trimmed flush to the external opening and secured with two interrupted 10-0 nylon sutures (Fig. 2). Next, the plate of the AGV was explanted from the underlying sclera. The conjunctiva was drawn back in place and sutured. For implantation of a new AGV, a fornix-based incision was made through the conjunctiva and the Tennon's capsule at the superonasal quadrant. A pocket was created between the superior rectus and the medial rectus muscles by blunt dissection of the Tennon's capsule from the episclera. A new valve body was inserted into the pocket with its leading edges 8 mm posterior to the limbus. The tube of the valve was trimmed to permit a 2 mm insertion into the anterior chamber. A paracentesis was performed, and the anterior chamber was entered at the limbus with a sharp 23 G needle, parallel to the iris. The drainage tube was inserted through the needle track. The tube was secured to the sclera with two 10-0 Nylon sutures, and covered with pericardium (Tutoplast®). The conjunctiva and the Tennon's capsule were closed with 10-0 Nylon sutures.
The IOP ranged from 8 to 20 mmHg during the postoperative one month. At postoperative 14 months, IOP was 18 mm Hg and no fistula-related complication such as wound dehiscence or pericardium melting was noted.
Case 2: A 61-year-old man with diabetes mellitus, hypertension and NVG (LE) had undergone AGV implantation (LE) 19 months prior to its explantation. At 4 months postoperative, exposure of the AGV tube was noted. Except for mild hyperemia around the exposed tube, no significant inflammatory sign was noted in the anterior chamber or around the tube. IOP stayed under 20 mm Hg. However, the cataract of his left eye worsened enough to preclude a view of the fundus. Nineteen months after the AGV implantation, phacoemulsification, posterior chamber lens (PCL) implantation, AGV explantation and trabeculectomy with an intraoperative application of Mitomycin-C (0.2 mg/ml for 2 minutes) were performed concurrently on his left eye. The AGV was explanted by the method described previously. The IOP ranged from 9 to 23 mmHg during the early postoperative 3 months. At the latest follow-up at postoperative 23 months, the IOP was 13 mm Hg and no fistula-related complication was found (Fig. 3).
Case 3: A 56-year-old diabetic man who had undergone pars plana vitrectomy, phacoemulsification, PCL implantation and AGV implantation (LE) complained of ocular pain at postoperative 2 years. Hypopyon and exposure of AGV tube were noted. The patient was immediately treated with a topical fluoroquinolone (ciprofloxacin) hourly for presumed endophthalmitis. The next day the AGV was explanted using the aforementioned method. With topical antibiotics, the anterior chamber reaction resolved completely. No cultures yielded any organisms. However, at the postoperative day one, the IOP went up to the 40s. Diode laser transscleral cyclophotocoagulation was performed to control IOP. At postoperative 12 months, and the IOP was in the late 10s without any complications (Fig. 4).
Case 4: A 17-year-old man, who had undergone trabeculotomy, trabeculectomy and AGV implantation (LE) for congenital glaucoma, underwent a second AGV implantation (LE). Two months postoperatively, the tube of the second AGV was noted exposed. Explantation of the second AGV was performed using the pericardial plug in the aforementioned manner. The next day after the surgery, the IOP rose to 28 mmHg. Two hypotensive medications (Cosopt® and Alphagan-P®) were added postoperatively. During the postoperative 26 months, the anterior segment remained stable with IOP under control (Table 1).
Transconjunctival tube erosion is an infrequent but well-known complication of GDIs surgery. It is estimated that 2~7% of patients undergoing GDIs procedure develop melting of the overlying scleral or pericardial patch with erosion of the tube through the conjunctiva.8-13 Possible causes of conjunctival erosion include mechanical abrasion of the conjunctiva by the lid, excessive conjunctival tension over the tube, tube malposition, or lack of a smooth and tapered surface between the patch graft and the host along with poor ocular lubrication.10 Patch grafts like sclera, fascia lata, or dura mater may thin or disappear after implantation, secondary to ocular inflammation or a host-graft reaction.10 Pericardial patch grafts, which are supposed to be cell-free and without any antigenic stimuli, have also been reported to melt.4,10
Management of the exposed tube includes conjunctival autograft, scleral patch graft, amniotic membrane patch graft, and removal of the tube.3-6 If the tube exposure is accompanied by the infectious inflammatory signs which do not respond to antibiotics, the tube and the valve plate should be removed to help stop the propagation of the infectious process and prevent the subsequent development of endophthalmitis. Although the explantation of a GDIs is a simple procedure, it leaves an opening in the cornea and sclera that may not self-seal. If the GDIs has been implanted for a long period of time, it can be difficult to close the fistula which has been occupied by the tube.7 Mere direct sutures may close the defect. However, this would require a great amount of tissue tension and thus induce a significant amount of astigmatism which may be bothersome to an eye with good visual potential. Even if the fistula is sealed tight initially, there is still a chance that the fistula may recur later and lead to aqueous leakage if the suture is not strong enough to withstand the tissue tension. Therefore, tissue replacement may be an effective method to seal off the fistula, because it induces much less astigmatism postoperatively. Since the pericardium is highly biocompatible and provides an immediate and permanent seal, we used a pericardial plug to replace the corneoscleral fistula when explanting the AGV in all four of our cases.
Since Sibayan et al.7 first description of this surgical technique, there has been no published report of utilization of this method and its postoperative results. In Sibayan et al. study, they presented only the postoperative fourth week outcome of a single case. Our present study demonstrates the postoperative results of four cases that were followed up for a longer period. No fistula-related complication such as wound inflammation, leakage, or pericardium melting has been noted in any of our cases till the latest postoperative follow-up ranging from 12 to 26 months. Although the number of cases in our study is fairly small and the follow-up period is still short to verify the efficacy and stability of the method, the preliminary data of our cases may give support to the use of pericardium plug in such aforementioned conditions.
In conclusion, the pericardium plug seems to be an effective method in the repair of corneoscleral fistulas resulting from explantation of glaucoma valve implants. However, a prospective study of a larger number of cases with a long-term follow-up will be needed to ensure the stability of this method.
Figures and Tables
Fig. 1
Exposure of the tube and the body of the preexisting Ahmed Glaucoma Valve is shown with complete melting of the pericardium patch graft. The body of the AGV was located 4 mm posteriorly to the limbus.
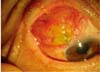
Fig. 2
Schematic illustration of the pericardium plug (black arrow) which is secured with 10-0 Nylon sutures (white arrow) to seal the defect previously occupied by the silicone tube of Ahmed glaucoma valve.
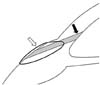
Fig. 3
(A) Slit-lamp photo shows the tube exposed externally through overlying conjunctival erosion. (B) Slit-lamp photo shows the pericardium plugged (black arrow) in the corneal fistula at 12 months after AGV explantation.

Fig. 4
(A) Slit-lamp photo shows the tube exposed externally through the overlying conjunctival erosion. Hypopyon is also noted. (B) Slit-lamp photo shows the pericardium plugged (black arrow) in the corneal fistula at 7 months after AGV explantation.

Table 1
Summary of the features of 4 cases in whom a pericardial plug was used to repair the corneoscleral fistula after Ahmed Glaucoma Valve had been explanted.

Dx=diagnosis; Preop=preoperative; Postop=at the last postoperative visit; Va=visual acuity; IOP=intraocular pressure; OP=operation; NVG=neovascular glaucoma; CG=congenital glaucoma; FC=finger count; HM=hand motion; PE=phacoemulsification and intraocular lens implantation; AGV=Ahmed glaucoma valve implantation; PPV=pars plana vitrectomy; Trab=trabeculectomy; CPC=cyclophotocoagulation.
References
1. Nguyen QH, Budenz DL, Parrish RK. Complications of Baerveldt glaucoma drainage implants. Arch Ophthalmol. 1998. 116:571–575.
2. Gedde SJ, Scott IU, Tabandeh H, et al. Late endophthalmitis associated with glaucoma drainage implants. Ophthalmology. 2001. 108:1323–1327.
3. Tsai JC, Grajewski AL, Parrish RK. Surgical revision of glaucoma shunt implants. Ophthalmic Surg Lasers. 1999. 30:41–46.
4. Lama PJ, Fechtner RD. Tube erosion following insertion of a glaucoma drainage device with a pericardial patch graft. Arch Ophthalmol. 1999. 117:1243–1244.
5. Ainsworth G, Rotchford A, Dua HS, King AJ. A novel use of amniotic membrane in the management of tube exposure following glaucoma tube shunt surgery. Br J Ophthalmol. 2006. 90:417–419.
6. Godfrey DG, Merritt JH, Fellman RL, Starita RJ. Interpolated conjunctival pedicle flaps for the treatment of exposed glaucoma drainage devices. Arch Ophthalmol. 2003. 121:1772–1775.
7. Sibayan SA, Latina MA. The use of processed pericardium in the repair of corneo-scleral fistulas. Ophthalmic Surg Lasers. 1997. 28(4):334–335.
8. Ayyala RS, Zurakowski D, Smith JA, et al. A clinical study of the Ahmed glaucoma valve implant in advanced glaucoma. Ophthalmology. 1998. 105:1968–1976.
9. Heuer DK, Lloyd MA, Abrams DA, et al. Which is better? One or two? A randomized clinical trial of single-plate versus double-plate Molteno implantation for glaucomas in aphakia and pseudophakia. Ophthalmology. 1992. 99:1512–1519.
10. Heuer DK, Budenz D, Coleman A. Aqueous shunt tube erosion. J Glaucoma. 2001. 10:493–496.
11. Huang MC, Netland PA, Coleman AL, et al. Intermediate-term clinical experience with the Ahmed glaucoma valve implant. Am J Ophthalmol. 1999. 127:27–33.
12. Coleman AL, Mondino BJ, Wilson MR, Casey R. Clinical experience with the Ahmed glaucoma valve implant in eyes with prior or concurrent penetrating keratoplasties. Am J Ophthalmol. 1997. 123:54–61.
13. Siegner SW, Netland PA, Urban RC Jr, et al. Clinical experience with the Baerveldt glaucoma drainage implant. Ophthalmology. 1995. 102:1298–1307.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download