Abstract
Purpose
To evaluate the efficacy and safety of fibrin bioadhesive in conjunctivolimbal autograft surgery for primary pterygium.
Methods
Thirty-six eyes in 34 patients were reviewed with nasal primary pterygium who were treated with pterygium excision with superior conjunctivolimbal transplantation with fibrin bioadhesive. Surgical durations were recorded and the patients were followed up on the first day after surgery and then at 1, 2, 4, 8 and 12 weeks postoperatively. The graft-recipient site attachments were examined and subjective symptoms of patients were recorded at every follow-up examinations.
Results
The mean patient age was 57.9±10.1 (ranging from 33 to 83) years. The mean follow-up period was 22.05±5.78 weeks. The mean surgery time was 18.04±5.65 minutes. The subjective symptoms (pain, foreign body sensation, tearing and discomfort) disappeared in 23 of 36 eyes (64%) in one week after surgery, and all discomforts subsided within two weeks after surgery in all patients. The conjunctivolimbal autograft was correctly positioned and fixed in 34 of 36 eyes (94.4%) throughout the follow-up period. Graft dehiscence was seen in two eyes (5.6%), one eye was treated with remedial sutures, and the other eye showed a spontaneous healing without remedial sutures. Transient graft edema occurred in four eyes (11.2%) but subsided spontaneously within a month. There were no cases of pterygium regrowth or complications due to the fibrin bioadhesive.
Conclusions
The use of fibrin bioadhesive in conjunctivolimbal autograft surgery in primary pterygium simplifies surgical techniques, shorten surgical duration, and produce less postoperative subjective symptoms. Therefore, the fibrin bioadhesive is a safe and effective tool to attach conjunctivolimbal autograft in primary pterygium surgery.
Pterygium, a common ocular surface disorder, is a triangular-shaped growth of bulbar conjunctival epithelium and hypertrophied subconjunctival connective tissue in the medialF and lateral palpebral fissure encroaching onto the cornea. Well-known risk factors are genetic predisposition, immune mechanism and chronic environmental irritations including ultraviolet rays, wind, and dusts, even though the etiology remains not clearly known.1 The prime challenge of pterygium surgery is prevention of recurrence. The reports of recurrence rate have discrepancies, but the recurrence rate of primary pterygium after simple excision is reportedly 25-45%.2 Subsequently, some techniques currently used to prevent recurrence of pterygium are beta-radiation, excimer laser, argon laser, Thio-Tepa and anti-metabolite drugs, conjunctivolimbal autograft and amniotic membrane graft.3-10
The recurrence of pteygium is closely associated with corneo-limbal stem cell deficiency. The recurrent pterygium is usually associated with more severe conjunctival inflammation, corneal involvement, and adhesion with surrounding tissue than the primary pterygium, causing conjunctival injection, ocular pain, ocular movement dysfunction and diplopia.
Anti-metabolites including mitomycin C (MMC) were introduced to prevent the recurrence of pterygium. The use of anti-metabolites is restricted by several complications including various ocular surface diseases, secondary glaucoma, and cataract.7
The transplantation of conjunctival or conjunctivolimbal autograft was reported to be the most effective method of lowering recurrence rate (2~9%) and occurrence of complications.9-11
However, the current method of attaching conjunctival or conjunctivolimal autograft by means of suturing presents several disadvantages, including complicated surgical techniques, prolonged operating time, prolonged postoperative patient discomfort, and suture-related potential complications. Moreover, many Asian patients, including Koreans, have narrower interpalpebral fissures than Caucasians or Africans, which can make suturing procedures more difficult to attach the conjunctival or conjunctivolimbal autograft.
Fibrin tissue bioadhesives which mimics the natural fibrinogen and thrombin reaction are used on various ophthalmologic surgeries for tissue adhesion, blood coagulation and wound healing.12-18 The use of tissue bioadhesives as an alternative means of attaching the grafts may shorten operating time, improve postoperative discomfort, and possibly avoid suture-related complications.16-18
The purpose of this study is to reveal the efficacy and safety of using fibrin bioadhesive (Tissucol Duo Quick®, Baxter AG, Vienna, Austria) in place of sutures during conjunctivolimbal autograft surgery for primary pterygium in Korean patients.
From March to October, 2006, 36 eyes in 34 patients (a consecutive case series) with nasal primary pterygium were treated with superior conjunctivolimbal transplant with fibrin glue (Tissucol Duo Quick®) at Cheil Eye Hospital by a single surgeon. Informed consent was obtained from all patients. Before surgery, a comprehensive investigation was taken including patient age, gender, medical and ocular history. Snellen visual acuity measurement, non-contact tonometry (Canon TX-10 tonometer), slit-lamp examination, and anterior segment photography were performed preoperatively. Patients with ocular pathology other than refractive error such as history of previous ocular surgery or trauma and known hypersensitivity to any component of fibrin bioadhesive were excluded.
The pterygia were graded according to the system used by Tan et al.11: grade 1 (atrophic), episcleral vessels under the body of the pterygium are not obscured and clearly distinguished; grade 3 (fleshy), episcleral vessels totally obscured; and grade 2 (intermediate), and all other pterygia not falling into these 2 grades (Table 1).
Fibrin glue (Tissucol Duo Quick®) is a tissue adhesive composed of two components which mimics the natural fibrin formation. One component contains fibrinogen mixed with coagulation factor 13 and aprotinin, and the other component contains thrombin and calcium chloride. Once the two components are mixed, fibrinogen is converted into fibrin by the action of thrombin; fibrin is then cross-linked by coagulation factor 13 to create a firm fibrin network. Aprotinin from bovine lungs prevent rapid fibrinolysis.
A single surgeon (Y.J. Park) performed all surgeries according to the same surgical techniques (Fig. 1). After instillation of topical proparacaine HCl (Alcaine®, Alcon Laboratories, Fort Worth, TX), the involved eye underwent standard ophthalmologic sterile preparation and draping. Then, the eye was exposed for operation using lid speculum. A lidocaine-epinephrine solution (Xylocaine® 2%, Astra-Zeneca, Sweden) was injected into the pterygium head to balloon out the conjunctiva. Blunt and sharp dissection was performed to separate the pterygium from the underlying sclera and surrounding conjunctiva. The pterygium head and surrounding atrophic conjunctival edges were excised and sclera was exposed. MMC was not used during or after the operation.
Before harvesting the free conjunctivolimbal autograft from superior limbus, the lidocaine-epinephrine solution was injected in to the donor conjunctiva to balloon out the area of the graft and separate it from the underlying Tenon's capsule. The donor graft at superior limbus was excised with an additional 1.0 mm of length and width relative to the dimensions of the graft bed. By use of minimal manipulation with atraumatic conjunctival forceps and Vannas scissors, the conjunctiva was carefully dissected away from the Tenon's capsule. Care was taken to prevent buttonholes and graft rollover. The dissected graft was flipped over the cornea, and then taking care that the palisades of Vogt was included in the graft, excision was made from limbal attachment. The free graft then was placed on top of the cornea and kept moist.
A drop of fibrinogen solution was placed on the bare sclera and spread out with a needle cannula. Thrombin solution was applied on the donor graft surface placed on top of the cornea. The graft coated with thrombin solution was then immediately flipped over and spread out onto the bare sclera coated with fibrinogen solution by using two McPherson forceps, and soon thrombin and fibrinogen reacted to seal the donor graft to bare sclera. Care was taken to ensure that the limbal stem cell population was oriented toward the limbus and the sides of the graft were opposed to the edges of the recipient conjunctiva. After a drying period of 5 minutes, the redundant margins of the graft were excised with Vannas scissors and the lid speculum was removed.
Neomycin sulfate/Polymyxin B sulfate/Dexamethasone ointment (Maxitrol® ointment, Alcon Laboratories, Fort Worth, TX) was applied to the involved eye and a pressure patch and a eye shield was kept on for 24 hours (Fig. 2).
Prednisolone acetate 1% (Pred-forte®, Allergan Inc., Irvine, CA) and 0.5% levofloxacin (Cravit® ophthalmic solution, Santen, Japan) eyedrops were applied 4 times daily for one month after the surgery.
To evaluate the efficacy of using fibrin bioadhesive in place of sutures during conjunctivolimbal autograft surgery for primary pterygium, operating time was recorded, the graft-recipient site attachment was observed and the subjective symptoms of patients were questioned in the postoperative follow-ups over 3 months. In order to assess the safety of this procedure, occurrence of complication was observed during follow-up examinations.
Operating time was measured starting from the placement of the lid speculum to its removal at the end of the surgery. Graft survival was defined as an intact graft by the fourth week after surgery, and graft failure was defined as the absence of the graft by the fourth week. Recurrence was defined as any growth of fibrovascular tissue into the cornea by slit lamp examination.
The patients were followed up on the first day after surgery and then on weeks 1, 2, 4, 8 and 12. Inquiries were made regarding the presence and duration of subjective symptoms including pain, foreign body sensation, tearing, and discomfort, at every follow-ups. Additionally, Snellen visual acuity measurement, non-contact tonometry (Canon TX-10 tonometer), slit-lamp examination, and anterior segment photography were performed. A slit-lamp examination was performed at every visit to monitor autograft-bed integrity and development of possible complications.
Of the 34 patients, 13 were male (38%) and 21 were female (62%). The mean patient age was 57.9±10.1 (SD) years (33-83 years). Of 36 eyes, 13 eyes were pterygium grade 2, and 24 eyes were grade 3 according to the grading system used by Tan et al.11 (Table 2)
All patients completed the 3-month follow-up period. The mean follow-up time was 22.05±5.78 (SD) weeks (Table 2). The mean surgery time was 18.04±5.65 (SD) minutes. One week after surgery, subjective symptoms including pain, foreign body sensation, tearing and discomfort had disappeared in 23 of 36 (64%) cases and all subjective symptoms subsided within two weeks for all cases.
In 34 of 36 (94.4%) cases, the conjunctivolimbal autograft was observed to be correctly positioned and fixed in all the follow-up exams (Fig. 2, 3). Graft dehiscence was observed in two eyes (5.6%), one eye was given remedial sutures, and the other eye showed spontaneous healing without remedial sutures after a week. Transient graft edema occurred in four eyes (11.2%) but spontaneously subsided within a month (Fig. 4). There were no cases of pterygium regrowth or complications due to the fibrin bioadhesive during the follow-up period.
Preventing pterygium recurrence is the main concern of pterygium surgery. The current major methods to prevent recurrence include use of MMC, conjunctival autografting, and amniotic membrane grafting.19 The meta-analysis of pterygium recurrence after surgery reported by Sanchez-Thorin et al.10 concluded that simple bare sclera resection alone is associated with 6 times higher odds of pterygium recurrence when MMC was used compared with conjunctival autograft, and 25 times higher odds of recurrence if MMC was not used. The authors, therefore, recommended that simple bare sclera excision should not be encouraged as a method of primary pterygium removal.10 Although the intraoperative use of MMC is effective in preventing pterygium recurrence, the use of MMC can be associated with sight-threatening complications such as corneoscleral melt, cataract, uveitis, secondary glaucoma, and symblepharon.3,7-10
According to several scientific papers so far, conjunctival autografting results in lower pterygium recurrence rates when compared with bare sclera excision9-11,20-27 and it is also associated with fewer complications compared to use of MMC.20 Only one case of necrotizing scleritis has been reported, and that case was successfully treated with steroid treatment.21 But, conjunctival autografting leaves conjunctival defects and adhesions due to fibrosis on the donor site, adding to the technical difficulty of another ocular surgeries, especially glaucoma surgery.2,22
Due to these problems, amniotic membrane transplantation has been suggested a replacement for conjunctival autografting in several studies. Amniotic membrane is the innermost layer of fetal membrane composed of thick basement membrane and monolayer epithelium. The amniotic membrane functions as a barrier to protect fetus from the maternal infection and immune mechanisms. In addition, amniotic membrane transplantation causes no rejections.28 The basement membrane of amniotic membrane promotes the differentiation and migration of epithelial cells and reinforce the attachment of basal epithelial cells.29 Several growth factors and anti-proteolytic factors from aminiotic membrane suppress the death and apoptosis of corneal surface cells;30 hence, it is used for the treatment of various ocular surface diseases. But in reality, amniotic membrane transplantation showed no reduction of recurrences in pterygium surgery compared to simple bare sclera technique. Amniotic membrane transplantation also had higher recurrence rate of pterygia compared to conjunctival autografting according to several studies.9,19,26,31
As stated, conjunctival autografting is safe and clearly effective method in preventing pterygium recurrence, but in addition to the donor site conjunctival defects, the technical difficulty of attaching the autograft and prolonged operation time with suturing can be a challenge for many surgeons.11,20-22 Furthermore, suture use is associated with patient discomfort (foreign body sensation, pain, discomfort, and tearing) and minor complications such as dellen ulcer, symblepharon, and graft dehiscence.18
The transplantation of autologous conjunctiva can be divided into the simple conjunctival autograft and the conjunctivolimbal autograft including the corneal limbus. Guler et al.23 reported a 13.3% recurrence rate in the seven cases followed for 14 to 24 months after conjunctivolimbal autografting in recurrent pterygium. The authors hypothesized the merit of conjunctivolimbal autografting including palisades of Vogt superior to simple conjunctival autografting in histologic structure reconstruction. Polute et al.24 reported the efficacy of conjunctivolimbal autografting in preventing the recurrence of pterygia the first several years after long-term follow-up study over an average 44.97 months. Kim et al.22 reported a 1.9% recurrence rate 15 months after conjunctivolimbal autografting without MMC use in patients under age 40 with primary pterygium. Ahn et al.25 reported no recurrences for 5 to 16 months after conjunctivolimbal autografting combined with the use of MMC in 10 cases of recurrent pterygium. This study also showed no recurrence of pterygium for 22.0±5.8 weeks follow-up after conjunctivolimbal autografting including the palisades of Vogt. Despite the relative brevity of follow-up period, there was no recurrence of pterygium and no differences in recurrence rate according to the pterygium grade. Nevertheless, more studies are required to prove clinical merits of superiority of conjunctivolimbal autografting in lowering recurrence of pterygium to simple conjunctival autografting in tissue reconstruction.
Biologic adhesives, such as fibrin glue, can be an alternative method of conjunctival or conjunctivolimbal graft attachment that may produce fewer complications and less postoperative discomforts. Fibrin glue has previously been used in ophthalmology for conjunctival wound closure, cataract surgery, oculoplastic and orbital surgery, repair of leaking glaucoma filtering blebs, lamellar keratoplasty, and attachment of amniotic membrane patch.12-18
Cohen and MacDonald16 have reported the utility of an organic tissue adhesive (Tisseel®, Baxter, Vienna, Austria) used to reduce the number of sutures needed for attaching conjunctival grafts in pterygium surgery. Koranyi et al.17 reported a randomized clinical trial that demonstrated that fibrin glue (Tisseel®) alone without suture can be used successfully to attach conjunctival autografts and at the same time reduce operating time and postoperative discomforts compared with vicryl 7-0 sutures. Harvey et al.18 also reported a randomized clinical trial to show the advantage of fibrin glue (Beriplast P®, Aventis Behring, King of Prussia, PA) compared to suturing with nylon 10-0 for attaching conjunctival limbal autograft after pterygium excision. No sight-threatening complications developed, and none of the eyes lost vision in both series. Vicryl 7-0 sutures were compared with fibrin glue in the study by Koranyi et al.17 and nylon 10-0 sutures compared with fibrin glue in the study by Harvey et al.18 fewer postoperative symptoms were reported when fibrin glue was used for attaching conjunctival autografts in both studies.17,24 These reports showed that grafts attached with fibrin glue are better tolerated than grafts attached with suturing.
The advantages of using fibrin glue for attaching graft include ease of use, shorter operating times, and less postoperative discomforts. This study showed the reduction of mean operation time compared to that of conjunctival or conjunctivolimbal autografting attached with suturing in other studies (Table 3). A recent study by Ti et al. reported that the success rate of sutured conjunctival or conjunctivolimbal autograft can vary widely among different surgeons (range, 5%-82%).21 This variability was probably attributed to different surgical skill levels on learning curves among different ophthalmologists. Because the use of fibrin glue removes the need for the tedious suturing process, the learning curve can be shortened, and better results may be more consistently achieved despite differences in surgical expertise. Moreover, conjunctival or conjunctivolimbal autografting will be better accepted by the patients, because the use of fibrin glue produces less subjective symptoms as shown when comparing of postoperative subjective symptoms in other studies (Table 4). In this study, only inquiry regarding the presence and duration of subjective symptoms (foreign body sensation, pain, discomfort, and tearing) was made, not 5-point scale self-evaluation used in studies by Harvey et al.18 due to the poor communication capability of a considerable numbers of the subjects. If 5-point scale self-evaluation was adopted in this study, more objective results could have been expected.
All autografts were successfully attached in this study. Wound gapping in two eyes (5.6%) and transient graft edema in four eyes (11.2%) were observed, but no critical complications due to fibrin glue were observed including corneal defects, symblepharon, giant papillary conjunctivitis, granulation and contact dermatitis, previously proposed in other studies as possible complications.11,20,22
There are some concerns regarding the safety of fibrin glue use, including potential for anaphylactic reaction and disease transmission.18 None of the patients in this study had anaphylactic reactions. Adherence to strict manufacturing processes can prevent transmission of pathogens.
In summary, fibrin glue is an effective and safe method for attaching conjunctival or conjunctivolimbal autografts during pterygium surgery. The use of fibrin glue can ease the surgical procedures, shorten operating times and produce less postoperative symptoms and discomfort. However, long-term postoperative studies are needed to confirm whether the rate of pterygium recurrence and other complications are affected by the use of fibrin glue.
Figures and Tables
Fig. 1
Surgical procedures of conjunctivolimbal autograft using a fibrin adhesive (Tissucol Duo Quick®) in pterygium surgery. (A) Pre-operative fleshy nasal pterygium. (B) The pterygium head was excised, the conjunctiva allowed to retract, and the subconjunctival fibrovascular tissue excised en bloc. (C) Superior conjunctivolimbal autograft was flipped over the cornea and the limbal attachment, and them the head of the palisades of Vogt was cleaned with a blade. (D) Conjunctivolimbal autograft kept placed over the cornea. The graft was rotated toward the defect site, spreading the conjunctiva out, being mindful of orienting the limbal stem cell population toward the limbus. (E) The fibrin glue was applied over the bare sclera and conjunctivolimbal autograft; A drop of fibrinogen solution was placed on the bare sclera and a drop of thrombin solution was placed conjunctivolimbal autograft. (F) Conjunctivolimbal autograft spread over the bare sclera with the edges bonded to the surrounding health conjunctiva by using McPherson forceps.
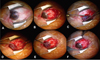
Fig. 2
The photograph anterior segment on day 1 after pterygium excision and superior conjunctivolimbal autograft attached with fibrin glue. Note the relatively low inflammatory appearance of the eye, well-positioned graft and reestablishment of the normal limbal architecture.
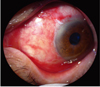
Fig. 3
Left column (A, B, C, D, E, F): Images of preoperative fleshy grade 3 nasal primary pterygium. Right column (G, H, I, J, K, L): Images of the conjuntivolimbal autograft of the same patients on 12 weeks after surgery. Well-positioned graft and reestablishment of the normal limbal architecture are observed with no signs of recurrence in all subjects.
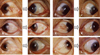
Fig. 4
(A) Mild shrinkage (between arrows) of the conjunctivolimbal autograft and recipient conjunctiva was noted 2 weeks after surgery: Secondary epithelialization and spontaneous closure of the wound gap occurred in one week. (B) Transient edema (arrows) of conjunctivolimbal autograft 2 weeks after surgery: Spontaneous resolution occurred in one month.
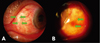
Table 2
Demographic charatacteristics of subjects (mean±SD)

*The pterygia were graded according to the system used by Tan et al.11
References
1. Lee SH, Jeong HJ. Immune reactions in pterygium. J Korean Ophthalmol Soc. 1987. 28:933–937.
2. Allan BDS, Short P, Crawford GJ, et al. Pinguecula and pterygia. Surv Ophthalmol. 1988. 33:41–49.
3. Amano S, Motoyama Y, Oshika T, et al. Comparative study of intraoperative mitomycin C and beta irradiation in pterygium surgery. Br J Ophthalmol. 2000. 84:618–621.
4. Talu H, Tasindi E, Ciftci F, Yildiz TF. Excimer laser phototherapeutic keratectomy for recurrent pterygium. J Cataract Refract Surg. 1998. 24:1326–1332.
5. Na KS, Kim JY, Choi GJ. A clinical observation on the argon laser effect of the pterygium. J Korean Ophthalmol Soc. 1996. 37:1120–1125.
6. Joselson GA, Muller VP. Incidence of pterygium recurrence in patients treated with Thio-tepa. Am J Ophthalmol. 1966. 81:891–895.
7. Panda A, Gopal KD, Suhas W, et al. Randomized trial of intaoperative mitomycin C in surgery for pterygium. Am J Ophthalmol. 1998. 125:59–63.
8. Rubinfeld RS, Pfister RR, Stein RM, et al. Serious complications of topical mitomycin-C after pterygium surgery. Ophthalmology. 1992. 99:1647–1654.
9. Ma DH, See LC, Liau SB, Tsai RJ. Aminotic membrane graft for primary pterygium: comparision with conjunctival autograft and topical mitomycin C treatment. Br J Ophthalmol. 2000. 84:973–978.
10. Sanchez-Thorin JC, Rocha G, Yelin JB. Meta-analysis on the recurrence rates after bare sclera resection with and without mitomycin C use and conjunctival autograft placement in surgery for primary pterygium. Br J Ophthalmol. 1998. 82:661–665.
11. Tan DT, Chee SP, Dear KB, Lim AS. Effect of pterygium morphology on pterygium recurrence in a contolled trial comparing conjunctival autografting with bare sclera excision. Arch Ophthalmol. 1997. 115:1235–1240.
12. Lagoutte FM, Gauthier L, Comte PR. A fibrin sealant for perforated and preperforated corneal ulcers. Br J Ophthalmol. 1989. 73:757–761.
13. Mandel MA. Closure of blepharoplasty incisions with autologous fibrin glue. Arch Ophthalmol. 1990. 108:842–844.
14. Kajiwara K. Repair of a leaking bleb with fibrin glue. Am J Ophthalmol. 1990. 109:599–601.
15. Kaufman HE, Insler MS, Ibrahim-Elzembely HA, Kaufman SC. Human fibrin tissue adhesive for sutureless lamellar keratoplasty and scleral patch adhesion: a pilot study. Ophthalmology. 2003. 110:2168–2172.
16. Cohen RA, McDonald MB. Fixation of conjunctival autografts with an organic tissue adhesive. Arch Ophthalmol. 1993. 111:1167–1168.
17. Koranyi G, Seregard S, Kopp ED. Cut and paste: a no suture, small incision approach to pterygium surgery. Br J Ophthalmol. 2004. 88:911–914.
18. Harvey SU, Reyes JM, Flore JD, Lim-Bon-Siong R. Comparison of fibrin glue and sutures for attaching conjunctival autografts after pterygium excision. Ophthalmology. 2005. 112:667–671.
19. Kim JC, Lee DH, Shyn KH. Clinical use of human amniotic membrane for ocular surface diseases. Advances in Corneal Research. 1997. 117–134.
20. Sridhar MS, Bansal AK, Rao GN. Surgically induced necrotizing scleritis after pterygium excision and conjunctival autograft. Cornea. 2002. 21:305–307.
21. Ti SE, Chee SP, Dear KB, Tan DT. Analysis of variation in success rates in conjunctival autografting for primary and recurrent pterygium. Br J Ophthalmol. 2000. 84:385–389.
22. Kim YS, Kim JH, Byun YJ. Limbal-conjunctival autograft transplantation for the treatment of primary pterygium. J Korean Ophthalmol Soc. 1999. 40:1804–1810.
23. Guler M, Sobaci G, Liker S, et al. Limbal-conjunctival autograft transplantation in cases with recurrent pterygium. Acta Ophthalmologica. 1994. 72:721–726.
24. Polute P, Heilinggenhaus A, Koch J, Steuhl KP. Long-term results of autologous conjunctival-limbus transplantation in pterygium. Klin Monatsbl Augenheilkd. 1998. 213:9–14.
25. Ahn DG, Auh SJ, Choi YS. The clinical results of limbal conjunctival transplantation with intraoperative mitomycin C application for recurrent pterygium. J Korean Ophthalmol Soc. 1999. 40:2443–2449.
26. Park JM, Ahn HB, Park WC. The effect of combined amniotic membrane and limbal transplantation for recurrent pterygium or pseudopterygium. J Korean Ophthalmol Soc. 2003. 44:1504–1511.
27. Oh TH, Choi GY, Yoon BJ. The effect of conjunctival autograft for recurrent pteygium. J Korean Ophthalmol Soc. 1994. 35:1335–1339.
28. Van Herendael BJ, Oberti C, Brosens I. Microanatomy of the human amniotic membrane: a light microscopic, transmission, and scanning microscopic study. Am J Obstet Gynecol. 1978. 131:872–880.
29. Terranova VP, Lyall RM. Chemotaxis of human gingival epithelial cells to laminin: a mechanism for epithelial cell apical migration. J Periodontol. 1986. 57:311–317.
30. Na BK, Hwang JH, Kim JC, et al. Analysis of human amniotic membrane components as proteinase inhibitors for development of therapeutic agent for recalcitrant keratitis. Tropho Res. 1999. 13:453–466.
31. Kim MJ, Tchah HW. Treatment of pterygium with amniotic membrane transplantation. J Korean Ophthalmol Soc. 1998. 39:59–64.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download