Abstract
Purpose
To compare the levels of calcium and phosphorus in the aqueous humor and serum of diabetics and non-diabetics.
Methods
We divided patients into two groups: seventy-six non-diabetic cataract patients and fifty-two diabetic cataract patients. The diabetic group was divided again into three subgroups: twenty-six patients with no diabetic retinopathy, thirteen patients with non-proliferative diabetic retinopathy, and thirteen patients with proliferative diabetic retinopathy. The authors compared the levels of calcium and phosphorus in the serum and aqueous humor of cataract patients. Statistic analysis was performed to form two comparisons: 1) a comparison between non-diabetics and diabetics and 2) a comparison among non-diabetics and the three subgroups of diabetics.
Results
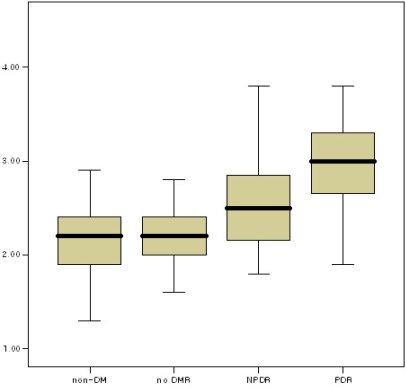
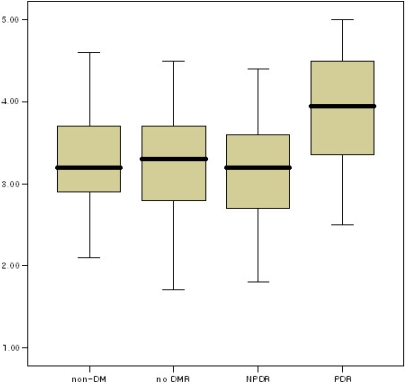
In serum, calcium levels did not statistically differ between non-diabetics and diabetics. The phosphorus level was also not significantly different. In the aqueous humor, however, while calcium levels did not differ significantly, the phosphorus levels in diabetics were considerably higher than those in non-diabetics. When non-diabetics were compared to the three diabetic subgroups, calcium levels did not differ in serum or aqueous humor, but the phosphorus levels in diabetics with proliferative diabetic retinopathy were significantly higher than those in non-diabetics, diabetics without diabetic retinopathy, and diabetics with non-proliferative diabetic retinopathy.
Conclusions
The level of phosphorus in the aqueous humor and serum of diabetics was significantly increased, especially in diabetics with proliferative diabetic retinopathy. This result may be related to hydrophilic acrylic IOL opacification. Future studies regarding the pathogenic role of a high concentration of aqueous humor and serum phosphorus are required.
A common and effective treatment for cataracts involves the implantation of a foldable intraocular lens (IOL) through a small incision after phacoemusification of the cataract. The foldable IOL is often composed of hydrophilic acryl, which is soft, autoclave sterilizable, hydrophilic, and has superior biocompatibility compared with PMMA (polymethymethacrylate), silicone, and hydrophobic acryl.1-6 However, the hydrophilic acryl hydrogel IOL is the most common type of lenses removed.7 In 93% of these cases, the IOL had to be removed due to late opacification of the optic area of the lens.7 Cases involving the opacification of hydrogel IOLs made from various materials and designs are steadily reported around the world.8-23 In many cases, such opacification resulted from the deposition of calcium and phosphorus inside the IOL, outside the IOL or both.8-20 Opacification is more frequently reported in diabetics, especially those with proliferative diabetic retinopathy.17,18,22,23 In this study, the authors compared levels of calcium and phosphorus in the aqueous humor and serum of non-diabetics and diabetics to investigate the basis for the increased incidence of late opacification of hydrophilic
acrylic intraocular lenses in diabetic patients.
The study included 128 patients who had undergone phacoemulsification with IOL implantation between Oct 23, 2003 and Feb 23, 2005 at Seoul Veterans Hospital. Only senile cataract patients without a prior intraocular surgery or history of complications like glaucoma or uveitis were enrolled. The average age was 68 years old (51-84 years old) and the ratio of males to females was 106 to 22. Fifty two patients were diabetic (40.6%), and among these 26 had no diabetic retinopathy (20.3%), 13 had non-proliferative diabetic retinopathy (10.2%) and 13 had proliferative retinopathy (10.2%).
Before surgery, we administered 2% lidocaine for local anesthesia, and then we made a temporal clear cornea incision with a 1.3 mm MVR blade and sampled 0.1~0.2 cc of aqueous humor with a 1 cc tuberculin syringe capped with an anterior irrigation tip instead of a needle. The levels of calcium and phosphorus in the sample were immediately measured with an automated analyzer (TBA-200 FR NEO biochemical analyzer, Toshiba) by the Department of Clinicopatholgy. In addition, a serum sample was obtained at admission, and the levels of calcium and phosphorous in this sample were also measured with this automated analyzer.
The data was statistically analyzed with SPSS Win 12.0, using a 95% level of significance. T-Tests were used to compare the average values of calcium and phosphorus in the aqueous humor of the diabetic and non-diabetic groups. A one-way ANOVA was used to compare the mean values of calcium and phosphorus among non-diabetics and the three diabetic subgroups (no diabetic retinopathy, non-proliferative diabetic retinopathy, and proliferative diabetic retinopathy).
The Tukey B approach of multiple comparison tests was used to identify any group that differed widely from the other groups. A bivariate correlation analysis and a linear regression analysis were used to investigate the relationship between the four factors: calcium and phosphorus in the aqueous humor and in serum.
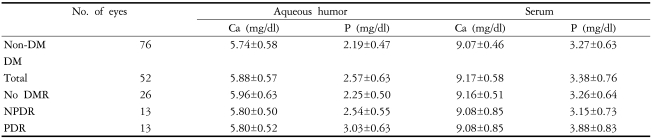
In the aqueous humor, the mean value of phosphorus in diabetics was 2.57±0.63 mg/dl, which was significantly higher than that in non-diabetics (2.19±0.47 mg/dl) (p=0.002), but there was no significant difference in calcium levels between diabetics and non-diabetics (p=0.19). In serum, neither calcium nor phosphorus differed significantly between the two groups (independent T-test, serum calcium p=0.253, serum phosphorus p=0.34).
When the non-diabetics were compared to the 3 subgroups of diabetics (diabetics without diabetic retinopathy, non-proliferative diabetic retinopathy, and proliferative diabetic retinopathy), phosphorus in the aqueous humor and serum differed notably, but calcium did not differ significantly in either fluid compartment (one-way ANOVA, aqueous humor phosphorus p=0.000, serum phosphorus p=0.02, serum calcium p=0.453, serum calcium p=0.476) (Table 1).
The mean values of serum phosphorus and aqueous humor phosphorus were not significantly different among non-diabetics, diabetics without diabetic retinopathy, and those with non-proliferative diabetic retinopathy. However, the mean value of serum and aqueous humor phosphorus in diabetics with proliferative diabetic retinopathy was much higher than that in the other three groups (Tukey B, aqueous humor phosphorus p<0.05, serum phosphorus p<0.05) (Fig. 1. and Fig. 2.).
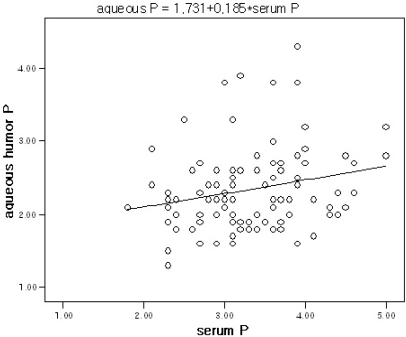
The level of aqueous humor phosphorus and serum phosphorus were significantly correlated to one another (bivariate correlation analysis, r=0.218, p=0.031), but were not correlated to calcium serum or aqueous humor levels. The level of aqueous humor phosphorus was related to the level of serum phosphorus by linear regression analysis, and the simple regression equation was determined to be: aqueous humor phosphorus=1.731+0.185 X (serum phosphorus) (linear regression analysis, F=4.817, p=0.031) (Fig. 3).
The opacification of hydrophilic acrylic foldable intraocular lenses has been reported in many cases around the world since Chang et al12's report, and possible causes for this opacification have been suggested.8-20 The opacifications are induced by hydroxyapatite, containing calcium and phosphorus, as demonstrated by von Kossa stain, Alizarin red stain, scanning Electron microscope (SEM), FR-IR spectrometer, X-ray photoelectron spectroscopy, and energy dispersive X-ray spectroscopy.8,16-20
Werner et al11 reported that the source of this excessive calcium was residual lens substance. In normal aqueous humor, calcium is found in low concentrations (about half of serum calcium), and an increase in these levels caused by intraocular inflammation, high calcium or phosphorus in intraocular drug administration, or intraoperative perfusates may cause dystrophic calcification. However, those intraoperative perfusates and viscoelastics have little direct relation to the opacification in question, since the opacification occurs long after the surgery and the materials are not used equally in all cases.
The intraocular lens itself has been considered important in the causation of IOL opacification. However, opacifications are not found in all patients operated on by the same surgeon, using the same surgical materials. Kim et al21 reported that opacification occurred in 70 out of 91 eyes (85.4%) selected from 69 patients observed for more than 6 months after implanting the ACRL-C160 intraocular lenses. In an unpublished study at our hospital, opacification occurred in 33 out of 91 eyes (39%) selected from 77 patients observed for about 23.2 months after implanting ACRL-C160 intraocular lenses. The authors could confirm that not all patients experience opacification, even though the incidence differed from that of Kim's report. Therefore, the IOL opacification cannot be attributed to the IOL alone, but may rely on other factors that alter the intraocular environment, such as an increase in the aqueous calcium and phosphorus or an acceleration of their deposition. Systemic diseases like diabetes and hypertension have been proposed as a major factor in the etiology of opacification. Specifically, diabetes mellitus has been reported as the most frequent cause of opacification among various systemic diseases. Groh MJ et al22 reported that 6 of 12 patients with IOL opacification had diabetes. Werner et al11 remarked that metabolic imbalance is an essential factor in the causation of IOL opacification, observing that most patients with IOL opacification also had cardiovascular disease, arthritis, hypertension, or diabetes. Neuhann et al23 reported that diabetes was the systemic disease most frequently accompanied by IOL in a study in which opacified IOLs were removed from 106 eyes. Lee et al17 considered diabetes a triggering factor for IOL opacification because all 4 patients that underwent IOL exchange had diabetes. Ha et al18 remarked that increased intraocular calcium levels may be a direct cause of IOL opacification considering that the patients in his study were all diabetics who had proliferative diabetic retinopathy for more than 10 years. In an unpublished study from our hospital, 18 out of 33 eyes (67%) selected from patients who had IOL opacification were accompanied by diabetes. However some reports suggest that IOL opacification is not significantly related to diabetes.20,21 In a study investigating the relationship between IOL opacification and systemic disease, Kim et al21 remarked that diabetes did not significantly affect the occurrence of opacification but that opacification progressed faster in patients with diabetes, especially in those that had long-standing disease. The opacification also appeared both accelerated and more severe in patients with proliferative diabetic retinopathy.
Several researchers have reported that IOL opacification occurred more frequently, faster, and more severely in the eyes of diabetics. They have also suggested that levels of aqueous humor calcium may be causatively related. Pombo et al24 extracted the vitreous of patients with proliferative diabetic retinopathy and proliferative vitreoretinopathy and found that the ATP released from blood vessels of the retina induced an increase in calcium. They also found that high levels of prorenin were found in patients with proliferative diabetic retinopathy, which produces angiotensin I and increases calcium levels. Some researchers reported that cytokine-like vascular endothelial growth factor (VEGF) increases the permeability of the blood-aqueous barrier and continuously promotes cell-mediated inflammation. The levels of albumin, glucose, and sulfur remained high, compared with normal eyes, due to the damaged blood-aqueous barrier present in diabetic retinopathy.25
The levels of aqueous humor calcium were not statistically different between diabetics and non-diabetics or among non-diabetics and the three subgroups of diabetics. This contradicted our expectation that increased permeability of the blood-aqueous barrier would increase aqueous humor calcium levels in patients with proliferative diabetic retinopathy. It is difficult to simply compare this study with Kim et al21's study since Kim et al compared serum calcium and phosphorus between an opacified IOL group and a non-opacified IOL group.
The high concentration of serum and aqueous humor phosphorus in diabetics with proliferative diabetic retinopathy is thought to result from renal insufficiency in diabetes mellitus. When high levels of phosphorus are maintained for long periods, the driving force for mineralization is increased, and calcium phosphate may be deposited in abnormal sites (ectopic calcification).26
Many articles report that high serum phosphate levels with normal calcium levels may influence vascular, prosthetic valvular, and extraskeletal calcifications by three separate mechanisms: by ectopic calcification resulting from hyperphosphatemia, by worsening secondary hyperparathyroidism, which in turn facilitates calcification, and by promoting calcium phosphate deposition in pre-formed endothelial plaques in the arterial wall.27,28
If increased aqueous humor and serum phosphorus may induce IOL opacification by promoting the formation of a calcium-phosphorus compound, our finding that aqueous humor and serum phosphorus were elevated in patients with proliferative diabetic retinopathy may explain the preponderance of patients with proliferative diabetic retinopathy that experience IOL opacification.
Future studies should explore the role of elevated serum and aqueous humor phosphorus in the pathogenesis of hydrophilic acrylic IOL opacification, especially in diabetics with proliferative diabetic retinopathy.
References
1. Barrett GD, Constable IJ, Stewart ED. Clinical results of hydrogel lens implantation. J Cataract Refract Surg. 1986; 12:623–631. PMID: 3783468.

2. Barrett GD, Constable IJ. Corneal endothelial loss with new intraocular lenses. Am J Ophthalmol. 1984; 98:157–165. PMID: 6476043.

3. Blumenthal M, Chen V. Soft intraocular lenses: evolution and potential. 1991. 1st ed. London: Wolfe;p. 122–124.
4. Carlson KH, Cameron JD, Lindstrom RL. Assessment of blood-aqueous barrier by fluorophotometry following PMMA, silicone and hydrogel lens implantation in rabbit eyes. J Cataract Refract Surg. 1993; 19:9–15. PMID: 8426331.
5. Percival SPB, Jafree AJ. Preliminary results with a new hydrogel intraocular lens. Eye. 1994; 8:672–675. PMID: 7867826.

6. Percival SPB. A prospective study of hydrogel and polymethylmethacrylate lenses. Dev Ophthalmol. 1989; 18:111–113. PMID: 2776940.
7. Mamalis N. Complications of foldable intraocular lenses requiring explantation or secondary intervention. J Cataract Refract Surg. 2002; 28:2193–2201. PMID: 12498859.
8. Werner L, Apple DJ, Kaskaloglu M, Pandey SK. Dense opacification of the optical component of a hydrophilic intraocular lens: a clinicopathological analysis of 9 explanted lenses. J Cataract Refract Surg. 2001; 27:1485–1492. PMID: 11566535.
9. Izak AM, Werner L, Pandey SK, Apple DJ. Calcification of modern foldable hydrogel intraocular lens designs. Eye. 2003; 17:393–406. PMID: 12724703.

10. Pandey SK, Werner L, Apple DJ, Kaskaloglu MM. Hydrophilic acrylic intraocular lens optic and haptics opacification in a diabetic patient: bilateral case report and clinicaopathological correlation. Ophthalmology. 2002; 109:2042–2051. PMID: 12414413.
11. Werner L, Apple DJ, Escobar-Gomez M, et al. Postoperative depostion of calcium on the surfaces of a hydrogel intraocular lens. Ophthalmology. 2000; 107:2179–2185. PMID: 11097592.
12. Chang BY, Davey KG, Gupta M, Hutchinson C. Late clouding of an acrylic intraocular lens following routine phacoemulsification. Eye. 1999; 13:807–808. PMID: 10707158.

13. Yu AK, Kwan KY, Chan DH, Fong DY. Clinical features of 46 eyes with calcified hydrogel intraocular lenses. J Cataract Refract Surg. 2001; 27:1596–1606. PMID: 11687358.

14. Habib Ne, Freegard TJ, Gock G, et al. Late surface opacification of Hydroview intraocular lenses. Eye. 2002; 16:69–74. PMID: 11913892.

15. Oner HE, Durak I, Saatci OA. Late surface opacification of hyderphilic acrylic intraocular lenses. Ophthalmic Surg Lasers. 2002; 33:304–308. PMID: 12134990.
16. Dorey MW, Brownstein S, Hill VE, et al. Proposed pathogenesis for the delayed postoperative opacification of the Hydroview hydrogel intraocular lens. Am J Ophthalmol. 2003; 135:591–598. PMID: 12719064.

17. Lee DH, Seo Y, Joo CK. Progressive opacification of hydrophilic acrylic intraocular lenses in diabetic patients. J Cataract Refract Surg. 2002; 28:1271–1275. PMID: 12106740.

18. Ha MS, Kang BD, Myung NH, et al. Postoperative deposition of calcium on the surface of hydrophilic acrylic IOL in diabetic patients. J Korean Ophthalmol Soc. 2002; 43:375–380.
19. Lee JY, Joo KM, Kim SH. Late opacification of hydrophilic acrylic intraocular lenses. J Korean Ophthalmol Soc. 2002; 43:2419–2429.
20. Frohn A, Dick HB, Augustin AJ, et al. Late opacification of the foldable hydrophilic acrylic lens SC60B-OUV. Ophthalmology. 2001; 108:1999–2004. PMID: 11713068.

21. Kim JC, Kim CS, Choi SH, et al. Clinical characteristics of patients with opacification of hydrophilic acrylic intraocular lens after cataract surgery. J Korean Opthalmol Soc. 2005; 46:1281–1290.
22. Groh MJ, Schlotzer-Schrehatdt U, Rummelt C, et al. Postoperative opacification of 12 hydrogel foldable lenses (Hydroview). Klin Monastsbl Augenheilkd. 2001; 218:465–468.
23. Neuhann IM, Werner L, Izak AM, et al. Late postoperative opacification of a hydrophilic acrylic intraocular lens. Ophthalmology. 2004; 111:2094–2101. PMID: 15522377.
24. Pombo C, Bokser L, Casabiell X, et al. Partial characterization of a putative new growth factor present in pathologic human vitreous. Graefes Arch Clin Exp Ophthalmol. 1996; 234:155–163. PMID: 8720714.
25. Wroblewski R, Zaczek A. X-ray microanalytical and morphological observations of aqueous humor from cataract human eyes with and without diabetes mellitus. J Submicrosc Cytol Pathol. 1999; 31:367–373. PMID: 10626005.
26. Isselbacher , Braunwald , Wilson , et al. Harrison's Principles of Internal Medicine. 1994. Vol. 2:13th ed. New York: Mcgraw-Hill Inc;p. 2140–2141.
27. Jono S, McKee MD, Murry CE, Shioi A, et al. Phosphate regulation of vascular smooth muscle cell calcification. Circ Res. 2000; 87:10–17.

28. Jorge B. Cannata-Andia and Minerva Rodriguez-Garcia. Hyperphosphatemia as a cardiovascular risk factor- how to manage the problem. Nephrol Dial Transplant. 2002; 17:16–19.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download