Abstract
Purpose
To evaluate visual outcome and the changes of contrast sensitivity (CS) after diffuse lamellar keratitis (DLK).
Methods
Using retrospective chart review, 48 eyes of 25 patients who underwent laser in situ keratomileusis (LASIK) with Visx S4 (VISX Inc., Santa Clara, CA) and M2 (Moria, France) and who were followed for at least six months were included. They were divided into two groups: DLK and non-DLK, by diagnosis of DLK or its absence after LASIK. Postoperative logMAR visual acuities and logCS measured using the VCTS® 6500 (Vistech Consultants, Inc., Dayton, OH) were compared with preoperative values in the DLK and non-DLK groups at three and six months after LASIK.
Results
There was no difference in logMAR visual acuity between the DLK and non-DLK groups until the sixth postoperative month. However, CS was significantly decreased at 12 and 18 cycle/degree compared with preoperative values (p=0.043 and p=0.045, respectively) in the DLK group, whereas CS was significantly increased at 12 cycle/degree in the non-DLK group (p=0.042) at six months.
Currently, some articles have reported that functional vision could be changed after laser in situ keratomileusis (LASIK).1-3 Optical quality changes could be one cause of the decrease in contrast sensitivity (CS) after LASIK.3 Diffuse lamellar keratitis (DLK), first reported in 1998,4 has been characterized as a noninfectious, inflammatory reaction in the lamellar interface shortly after LASIK.4-6 Recently, it was reported that DLK involved in the center might be associated with a visual loss.7 That is, one reason for functional deterioration in LASIK could be related to DLK. Therefore, we undertook this study to investigate the relationship of DLK with depressed CS after LASIK.
One hundred forty-four eyes were treated with LASIK in Seoul National University Hospital from January 2002 to March 2004. Of the 144 eyes, 48 (33%) were diagnosed with DLK. With the retrospective chart review, 48 eyes of 25 patients without ocular or systemic disease, followed-up for at least six months were included in this study. They were divided into two groups: DLK and non-DLK, by diagnosis of DLK or its absence after LASIK. The mean age of the patients was 26.0±6.3 years (range, 20 to 37 years).
All LASIK procedures were performed by one surgeon using the VISX STAR S4 (VISX Inc., Santa Clara, CA). Three drops of an anesthetic eyedrop (proparacaine hydrochloride 0.5%, Alcaine®, Alcon, Belgium) were topically instilled. An M2 microkeratome (Moria, Antony, France) was used to create a superior hinged 110 µm flap measuring 8.5 mm in diameter. After photoablation, the flap was replaced on the stromal bed and the interface was irrigated. The duration of the flap-lift period and of irrigation was measured. The flap was dried for three minutes with intermittent epithelial hydration using a wet Merocel sponge. Antibiotics (levofloxacin 0.5%, Cravit®, Santen, Japan), corticosteroids (fluorometholone acetate, Flarex®, Alcon, Belgium), and NSAIDs (diclofenac sodium, Decrol®, Il-yang, Korea) were topically instilled four times a day for the first week.
DLK was diagnosed after postoperative day one. Patients were followed-up at the following intervals: postoperative day one or two; at one, two, and four weeks; and two, three, and six months. The DLK was graded according to the classification proposed by Linebarger et al.6 in 2000: Grade 1, the presence of white granular cells in the periphery of the lamellar flap, outside the visual axis; Grade 2, the presence of white granular cells in the center of the flap, involving the visual axis, in the flap periphery, or in both; Grade 3, aggregation of more dense, white, and clumped cells in the central visual axis, with relative clearing in the periphery; and Grade 4, severe lamellar keratitis with stromal melting, permanent scarring, and associated visual morbidity.
To evaluate the effect of DLK on visual acuity and CS, preoperative and postoperative values of visual acuity and CS were compared in eyes with (n=22) and without (n=26) DLK. Best-corrected preoperative visual acuities (preoperative BCVA) were 20/25 or better. To compare the preoperative condition of the eyes with and without DLK, preoperative refractive error and amount of laser-ablated refractive power were reviewed. Postoperative BCVA and uncorrected visual acuity (UCVA) were measured using the Snellen visual acuity chart at one, three and six months postoperatively, and they were converted to logMAR (minimal angle of resolutions) visual acuity. CS was measured with Vistech VCTS® 6500 (Vistech Consultants, Inc., Dayton, OH) for five spatial frequencies: 1.5, 3, 6, 12 and 18 cycles/degree (cpd). LogMAR visual acuity and logCS at three and six months were compared with preoperative values in the DLK and non-DLK groups.
The statistical analyses were performed with SPSS 12.0 (SPSS Inc., Chicago, IL). A p-value less than 0.05 was considered statistically significant. The changes of logMAR visual acuity and logCS were compared using the paired t-test in each group, and with independent t-test between the two groups.
Of the 144 eyes overall, DLK developed in 48 eyes (33%): 41 (85%) in stage 1, 6 (13%) in stage 2, and 1 (2%) in stage 3. Forty-four eyes (91.7%) were diagnosed on the first or second postoperative day (range, 1-15 days) and 33.3 % of eyes with DLK were cured completely in a week (average 12.9 days; range, 4-40 days). Only one eye showed DLK on the fifteenth postoperative day, but there was no specific cause of delayed onset. Most DLK diagnoses included stage 1 or 2 and cleared rapidly with topical steroid eyedrop and oral steroid. One eye was stage 3, which was resolved after a two-week course of a topical steroid eyedrop (prednisolone acetate 1%, Pred forte®, Allergan, Belgium) every two hours, and an oral steroid.
Of the 144 eyes, 48 eyes met out inclusion criteria. There were 22 eyes in the DLK group and 26 eyes in the non-DLK group. The mean preoperative refractive error was -5.8±2.1 diopters (range, -1.75 to -10.0 diopters). That of the DLK and non-DLK group were -6.3±2.0 and -5.39±2.16 diopters, respectively, which was not statistically different (p=0.199, Mann-Whitney U test). The mean amounts of laser-ablated refractive power of the DLK and non-DLK groups were 6.4 ±1.8 and 5.6±2.2 diopters, respectively (p=0.342, Mann-Whitney U test).
Of the 48 eyes included in this study, mean preoperative logMAR BCVA of the DLK group was -0.021, which was not statistically different from that of the non-DLK group (-0.037, p>0.05, Mann-Whitney U test). Mean logMAR UCVA at one, three, and six months following LASIK was 0.001, 0.016, and -0.007 in the DLK group, and -0.035, -0.007, and -0.050 in the non-DLK group, respectively. There were no changes of logMAR UCVA up to six months, compared with the preoperative BCVA in each group (p>0.05, Wilcoxon signed rank test). Furthermore, there was no significant difference in postoperative UCVA between the two groups up to six months (p>0.05, Mann-Whitney U test, Fig. 1). The mean preoperative Snellen BCVA of five eyes with grade 2 DLK was 1.16, and the mean UCVA at postoperative one, three, and six months were 1.02, 0.84, and 0.93, respectively. The preoperative UCVA of the eye with grade 3 DLK was 0.8, and there were no visual acuity change more than two lines up to six postoperative months. Sixteen eyes with grade 1 DLK and 26 without DLK showed no significant difference in mean visual acuity preoperatively and postoperatively up to six months (p>0.05, Mann-Whitney U test).
There was no difference of CS in all spatial frequencies preoperatively between the DLK and non-DLK groups (p>0.05, Mann-Whitney U test). Three months after LASIK, however, logCS in the DLK group was decreased by 0.091 in 1.5 cpd, 0.028 in 3 cpd, 0.026 in 6 cpd, 0.251 in 12 cpd, and 0.112 in 18 cpd. The decrease of logCS in 12 cpd was statistically significant in the DLK group at three months postoperatively (p=0.023, Wilcoxon signed rank test). On the contrary, logCS in the non-DLK group was increased by 0.025 in 1.5 cpd, 0.033 in 3 cpd, 0.019 in 6 cpd, 0.028 in 12 cpd, and a 0.002 in 18 cpd, but none were statistically significant (p>0.05, Wilcoxon signed rank test) at three months (Fig. 2). Six months after LASIK, in the DLK group, there was a significant decrease of 0.277 in 12 cpd, 0.261 in 18 cpd (p=0.043, 0.045, Wilcoxon signed rank test, respectively), and insignificant changes with an increase of 0.010 in 1.5 cpd and a decrease of 0.196 in 3 cpd and 0.490 in 6 cpd. On the contrary, the changes of logCS in the non-DLK group showed marked increases of 0.043 in 1.5 cpd, 0.041 in 3 cpd, 0.127 in 6 cpd, 0.410 in 12 cpd, and 0.049 in 18 cpd, with the increase in 12 cpd being statistically significant (p=0.042, Wilcoxon signed rank test) (Fig. 3). When logCS of the grade 1 DLK eyes with that of the non-DLK eyes was compared, there was no significant difference in preoperative logCS (p>0.05, Mann-Whitney U test). But the decrease of logCS in 12 cpd was statistically significant at three and six months postoperatively (p=0.037 and 0.009, respectively, Mann-Whitney U test).
DLK is a syndrome characterized by multifocal lamellar infiltration to the flap interface after LASIK. Although it usually occurs on postoperative day one, it has occasionally been reported to developed up to six months after LASIK.4,5,8,9
About one third of the patients were diagnosed with DLK in the present study. Prior studies showed the incidence of DLK ranging from 0.4% to 29%.10-14 The causes of the high incidence of DLK found in our study are not exactly known. One possibility may be related to the use of microkeratome, which has a rotational torque movement and enables the construction of a thinner flap. The 110 head of M2 is designed to cut to a depth of 130 µm in the flap thickness. We think that this design may induce more superficial epithelial damage which can lead to DLK. In fact, diffuse superficial epithelial erosion was often found immediately after LASIK using slit lamp examination. Another possibility might be associated with the use of a plastic syringe to irrigate the interface. Although the effect of the use of the plastic syringe on DLK development could not be included in our study, we did find that the incidence of DLK was significantly reduced after replacing the plastic syringe with a silicone bulb (unpublished data). It is hypothesized that toxic substances may be released from these cheaper, domestic-made plastic syringes.
It is known that mild to moderate DLK does not affect the visual acuity several months after surgery.7,13,15 In our study, the postoperative BCVA was not significantly altered by DLK, at least up to six months. That is, there was no significant difference between the two groups at any time during the six month follow-up, although visual acuity was slightly lower in the DLK group than in the non-DLK group. This suggests that mild stromal changes with DLK do not affect the high contrast visual acuity. This may explain why a patient who complains of reduced visual performances in indoor activity after LASIK presents with normal Snellen visual acuity.
Meanwhile, although the visual environment is composed of objects with a variety of spatial frequencies and contrasts, high contrast visual acuity itself does not represent all of the visual performances in daily activities. Since CS testing was introduced by Shade16 in 1956, CS has been widely accepted to provide more information about functional vision than Snellen visual acuity.17,18 Currently, transient or permanent decreases in CS after LASIK have been reported.1-3,17,19-24 Although Moon and Tchah25 reported no CS decrease in all spatial frequencies, Cho and Kim26 described CS decrease in high spatial frequency up to 11 months postoperatively. Our study also showed a reduction of CS after LASIK up to six months in patients with DLK.
To assess CS change more accurately, logCS of the eyes with grade 1 DLK was reviewed to exclude the cases of visual acuity decrease due to stromal inflammation of the central cornea. After excluding the cases of grade 2 and 3 DLK, there was still logCS decrease in 12 cpd up to six months postoperatively. This fact means that inflammation of the peripheral cornea itself could cause CS decrease until six months postoperatively.
The exact reason for the decrease of CS after LASIK is still not clear, and the debate continues. Quesnel et al.20 suggested that subtle central corneal microstriae after LASIK cause the reduction of CS at medium to high spatial frequencies. Holladay et al.3 postulated that the change into oblate cornea from prolate cornea might worsen CS following LASIK due to increased high order aberration. Yamane et al.23 also proposed that increased ocular higher-order aberrations after LASIK compromise the postoperative CS function. Similar to the results of our study, Vajpayee RB et al.27 recently reported that the occurrence of intraoperative interface hemorrhage affects CS after LASIK.
We found decreased CS in nearly all spatial frequencies in LASIK with DLK, compared with that of preoperative eyes, at least up to six months. On the contrary, CS improved in 12 cpd at six months following LASIK in the non-DLK group. Therefore, we hypothesized that DLK might be strongly associated with the reduction of CS in LASIK. That is, decreased CS, especially in mid-spatial frequencies, may explain why some patients might experience inconvenience in daily life, such as with face recognition in low contrast.
CS might possibly be affected by flap-created stromal wound healing for three months. Therefore, CS in 12 cpd would be increased at six months (after completion of the stromal wound healing) in the non-DLK group, although there was no change of CS compared to that preoperatively until after three months. For the same reason, the depression of CS would be markedly expressed after allowing for the effect of the flap-created wound healing at six months.
DLK was significantly involved in the reduction of CS in LASIK up to six months postoperatively. Therefore, prevention of DLK development may be crucial to improve CS after LASIK.
Figures and Tables
Fig. 1
Comparison of the changes of visual acuity between the DLK and non-DLK groups following LASIK. The visual acuity of the non-DLK group was better at all times, but the difference was not statistically significant (p>0.05). MAR, minimal angle of resolutions; V/A, visual acuity; DLK, diffuse lamellar keratitis; preop, preoperative.

Fig. 2
Comparison of the changes of log contrast sensitivities (CS) three months following LASIK, compared to preoperative level. A: changes of logCS in diffuse lamellar keratitis (DLK) group after three months following LASIK. CS in 12 CPD was significantly decreased at three months postoperatively in the DLK group (*p=0.023, Wilcoxon signed rank test). B: changes of logCS in those without DLK. There were no changes in CS. CS, contrast sensitivity; DLK, diffuse lamellar keratitis; CPD, cycles per degree.
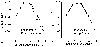
Fig. 3
Comparison of the changes of log contrast sensitivities six months following LASIK, compared to preoperative level. A: changes of log contrast sensitivity (CS) in diffuse lamellar keratitis (DLK) group after six months following LASIK. CS in 12 and 18 CPD were significantly decreased at six months postoperatively in the DLK group (*p=0.043, 0.045, respectively from left, Wilcoxon signed rank test). B: changes of logCS in those without DLK. CS in 12 CPD was significantly increased at six months postoperatively in the non-DLK group (†p=0.042, Wilcoxon signed rank test). CS, contrast sensitivity; DLK, diffuse lamellar keratitis; CPD, cycles per degree.

References
1. Chan JW, Edwards MH, Woo GC, Woo VC. Contrast sensitivity after laser in situ keratomileusis: one-year follow-up. J Cataract Refract Surg. 2002. 28:1774–1779.
2. Nakamura K, Bissen-Miyajima H, Toda I, et al. Effect of laser in situ keratomileusis correction on contrast visual acuity. J Cataract Refract Surg. 2001. 27:357–361.
3. Holladay JT, Dudeja DR, Chang J. Functional vision and corneal changes after laser in situ keratomileusis determined by contrast sensitivity, glare testing, and corneal topography. J Refract Surg. 1999. 25:663–669.
4. Smith RJ, Maloney RK. Diffuse lamellar keratitis: a new syndrome in lamellar refractive surgery. Ophthalmology. 1998. 105:1721–1726.
5. Kaufman SC, Maitchouk DY, Chiou AG, Beuerman RW. Interface inflammation after laser in situ keratomileusis. Sands of the Sahara syndrome. J Cataract Refract Surg. 1998. 24:1589–1593.
6. Linebarger EJ, Hardten DR, Lindstrom RL. Diffuse lamellar keratitis: diagnosis and management. J Cataract Refract Surg. 2000. 26:1072–1077.
7. Johnson JD, Harissi-Dagher M, Pineda R, et al. Diffuse lamellar keratitis: incidence, associations, outcomes, and a new classification system. J Cataract Refract Surg. 2001. 27:1560–1566.
8. Amano R, Ohno K, Shimizu K, et al. Late-onset diffuse lamellar keratitis. Jpn J Ophthalmol. 2003. 47:463–468.
9. Belda JI, Artola A, Alio J. Diffuse lamellar keratitis 6 months after uneventful laser in situ keratomileusis. J Refract Surg. 2003. 19:70–71.
10. Yuhan KR, Nguyen L, Wachler BS. Role of instrument cleaning and maintenance in the development of diffuse lamellar keratitis. Ophthalmology. 2002. 109:400–404.
11. Levinger S, Landau D, Kremer I, et al. Wiping microkeratome blades with sterile 100% alcohol to prevent diffuse lamellar keratitis after laser in situ keratomileusis. J Cataract Refract Surg. 2003. 29:1947–1949.
12. Thammano P, Rana AN, Talamo JH. Diffuse lamellar keratitis after laser in situ keratomileusis with the Moria LSK-One and Carriazo-Barraquer microkeratomes. J Cataract Refract Surg. 2003. 29:1962–1968.
13. Stulting RD, Randleman JB, Couser JM, Thompson KP. The epidemiology of diffuse lamellar keratitis. Cornea. 2004. 23:680–688.
14. Wilson SE, Ambrosio R Jr, Wilson SE, Ambrosio R Jr. Sporadic diffuse lamellar keratitis (DLK) after LASIK. Cornea. 2002. 21:560–563.
15. Hoffman RS, Fine IH, Parker M. Incidence and outcomes of LASIK with diffuse lamellar keratitis treated with topical and oral corticosteroids. J Cataract Refract Surg. 2003. 29:451–456.
16. Schade OH Sr. Optical and photoelectric analog of the eye. J Opt Soc Am. 1956. 46:721–739.
17. Perez-Santonja JJ, Sakla HF, Alio JL. Contrast sensitivity after laser in situ keratomileusis. J Cataract Refract Surg. 1998. 24:183–189.
18. Jindra LF, Zemon V. Contrast sensitivity testing: a more complete assessment of vision. J Cataract Refract Surg. 1989. 15:141–148.
19. Mutyala S, McDonald MB, Scheinblum KA, et al. Contrast sensitivity evaluation after laser in situ keratomileusis. Ophthalmology. 2000. 107:1864–1867.
20. Quesnel NM, Lovasik JV, Ferremi C, et al. Laser in situ keratomileusis for myopia and the contrast sensitivity function. J Refract Surg. 2004. 30:1209–1218.
21. Montes-Mico R, Espana E, Menezo JL. Mesopic contrast sensitivity function after laser in situ keratomileusis. J Refract Surg. 2003. 19:353–356.
22. Montes_Mico R, Charman WN. Choice of spatial frequency for contrast sensitivity evaluation after corneal refractive surgery. J Refract Surg. 2001. 17:646–651.
23. Yamane N, Miyata K, Samejima T, et al. Ocular higher-order aberrations and contrast sensitivity after conventional laser in situ keratomileusis. Invest Ophthalmol Vis Sci. 2004. 45:3986–3990.
24. Langrova H, Derse M, Hejcmanova D, et al. Effect of photorefractive keratectomy and laser in situ keratomileusis in high myopia on logMAR visual acuity and contrast sensitivity. Acta Medica (Hradec Kralove). 2003. 46:15–18.
25. Moon CS, Tchah HW. Results of LASIK for high myopia. J Korean Ophthalmol Soc. 1998. 39:865–871.
26. Cho SW, Kim HM. Comparison of the clinical results of lensectomy and LASIK for high myopia. J Korean Ophthalmol Soc. 1998. 39:1697–1706.
27. Vajpayee RB, Balasubramanya R, Rani A, et al. Visual performance after interface haemorrhage during laser in situ keratomileusis. Br J Ophthalmol. 2003. 87:717–719.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download