Abstract
Purpose
To report a case of conjunctival inclusion cysts on the corneal limbus of a patient with chronic vernal keratoconjunctivitis during 16 months' follow up
Methods
The patient was a 26 year old male without any specific history of surgery or trauma. Giant papillae, shield ulcers, and Horner-Trantas dots were detected. During the 16 month follow-up, Sodium cromoglycate eye drops and Prednisolone acetate 1% eye drops were given 3 times a day. During this period, conjunctival cysts were detected on the corneal limbus in both eyes. In spite of improvement of the corneal and conjunctival conditions, the conjunctival cysts did not seem to show any specific changes. For relief of foreign body sensation, excision of the conjunctival cysts and giant papillae of the left eye and histopathologic examination of the specimen was performed.
Vernal keratoconjunctivitis is a type of chronic conjunctivitis which develops, in most cases, before the age of 10, and usually persists for 2 to 10 years. It may be associated with allergic diseases such as atopy, asthma and eczema. Symptoms include severe itching sensation, foreign body sensation, mucoid discharge, glare, and conjunctival injection.1 The most typical findings of vernal keratoconjunctivitis are giant papillae with flat tops on the upper tarsal conjunctiva, Horner-Trantas dots and neovascularization distributed along the corneal limbus, and superficial punctate keratitis, corneal erosions and shield ulcers.2
During a long-term follow up of a 26 year-old patient with chronic vernal keratoconjunctivitis without a special history of trauma or surgery, conjunctival cysts were discovered on the corneal limbus in both eyes. The cysts were not noticed until 16 months after treatment. The size of the cysts appeared to be in proportion with the size of the corresponding giant papillae. After excision and histopathologic examination of the conjunctival cysts, the cysts were diagnosed as conjunctival inclusion cysts.
Therefore, we report this case as an atypical finding of chronic vernal keratoconjunctivitis.
In January of 2003, a healthy 26 year old male without any history of surgery or trauma visited our hospital for deterioration of vision and persisting pain in both eyes, which started one week before the visit. The corrected vision of both eyes at the time of visit was 20/40 in each eye. The patient did not have any prior allergic symptoms prior to this visit to the ophthalmologic clinic. On slit lamp examination, giant papillae in the upper tarsal conjunctiva (Fig. 1), persistent epithelial defects and shield ulcers, Horner-Trantas dots and neovascularization in the corneal limbus were detected in both eyes. A diagnosis of vernal keratoconjunctivitis was made, and the corneal shield ulcer was treated with Sodium cromoglycate eye drops (Opticrom®, Sanofi-aventis, France) and Prednisolone acetate 1% eye drops (Pred forte®, Allergan, USA) 3 times a day. Even though there was improvement of the overall conditions of the cornea and conjunctiva and improvement of corrected vision to 20/25 in the right eye and 20/30 in the left eye, the giant papillae did not show any specific changes. In May 2004, at the 16 month period of follow up, conjunctival cysts that had not been detected previously were observed in the corneal limbus of both eyes (Fig. 2). Even with Sodium cromoglycate eye drops and Prednisolone acetate 1% eye drops 3 times a day, conjunctival cysts did not disappear. The size of the cysts was larger in the left eye compared with the right, corresponding with the size of giant papillae, which was also larger in the left eye.
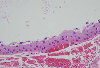
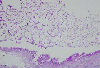
The size of conjunctival cysts was not changed and the patient complained of foreign body sensation and photopsia continuously especially in the left eye. We decided to remove the conjunctival cysts with the patient's consent, to relieve the symptoms and to know the nature of cyst. In March 2006, under local anesthesia, the conjunctival cysts and contacting giant papillae of the left eye were excised and histopathologic examinations were performed. The light microscopic findings showed that the conjunctival cysts were inclusion cysts. The walls were formed with non-keratinizing stratified epithelium and were filled with PAS-positive mucoid materials. Inflammatory cells were not found in the vicinity (Fig. 3, 4, 5). The contacting giant papillae showed chronic inflammatory changes.
Inclusion cysts are benign cysts filled with clear serous fluid containing shed cells or gelatinous mucous materials. The fluid is located within cyst walls, which consist of several layers of non-keratinizing epithelium and connective tissue. Twenty-five percents of acquired epithelial lesions of the conjunctiva, and 80% of the entire cystic lesions of the conjunctiva are inclusion cysts. The ratio of development is equal between male and female, and the mean age of onset is 47 years.3
Inclusion cysts are classified as primary or secondary, depending on their causes.4 The primary inclusion cyst is generally restricted to the superio-medial side of the orbit and is congenitally developed during the embryonal period by separation of a portion of conjunctival epithelial cells. The secondary type of inclusion cyst is more prevalent than the primary cyst. It is an acquired type of cyst and is located primarily in the supero-lateral side of the orbit. It occurs naturally or under inflammatory conditions of the conjunctiva, and it may be developed by amalgamation of mucosal folds. In most cases, it is developed by detachment of a portion of the conjunctival epithelium by surgery or trauma and its following implantation onto the conjunctival epithelium.
Cases of secondary inclusion cysts developed in the conjunctiva after ophthalmic surgery have been reported.5-7 Two cases of conjuctival cysts developed in patients without specific history of surgery, trauma or inflammation were reported by Srinivasan et al.8 Kiratli et al. have reported cases in association with pterygium as examples of inclusion cysts developed in the conjunctiva in association with chronic inflammation.9 Suzuki et al. have reported 2 cases of inclusion cysts in patients of age 8 and 9 with vernal keratoconjunctivitis without any clinical or morphologic findings.10 The infiltration of inflammatory cells were claimed to contribute to the development of inclusion cysts.
This case is an example of development of conjunctival inclusion cysts in a healthy 26 year old male after a 16 month follow-up of vernal keratoconjunctivitis.
Histopathologic section showed the conjunctival cyst lined with nonkeratinizing stratified epithelium filled with PAS positive ingredients with no inflammatory cells in the vicinity. The size of the conjunctival cysts was larger in the left eye than in the right, corresponding with the size of the giant papillae of the upper tarsus. It is possible that mechanical friction by the giant papillae of the upper tarsus had mediated an effect on the development of the inclusion cysts.11 A portion of the conjunctival epithelium could have been detached and had proliferated after being buried directly into the subepithelial connective tissue by mechanical stimulation. As a matter of fact, shield ulcers, which are characteristic findings of vernal keratoconjunctivitis, are developed by mechanical stimulation.12 In addition, histamine, eosinophil major basic protein, prostaglandin F, eosinophilchemotactic factor, tryptase and other characteristic inflammatory mediators of vernal keratoconjunctivitis may cause the development of inclusion cysts by inducing a certain toxic reaction.13,14 However, this is difficult to confirm, as immunological examinations of the excisional biopsy of the inclusion cysts were not performed in this case.
When the change of size of the inclusion cysts is minimal, no specific treatment is needed.15 In cases where the size is large, or there is foreign body sensation, corneal astigmatism, or impediment of sight, excision of the cyst, cryotherapy, electric cauterization, or YAG laser may be considered.16,17
In this case, excision of the conjunctival cysts and giant papillae of the left eye was performed because the cysts and papillae of the left eye were significantly larger than those of the right.
The conjunctival inclusion cysts might be an atypical finding of chronic vernal keratoconjunctivitis. The key factor of development of the cysts may be chronic irritative mechanical friction caused by giant papillae. Long standing giant papillae would be the inducing factor in the formation of conjunctival inclusion cysts in vernal keratoconjunctivitis.
Figures and Tables
Fig. 1
Giant papillae with flat tops on the upper tarsal conjunctiva (dot-arrow), typically described as a 'cobblestone' appearance were the hallmark of vernal keratoconjunctivitis and were different in shape compared with hypertrophic conjunctival cicatrization (lined-arrow). The size of the giant papillae in the left eye was larger than that of the right eye (A: Right eye, B: Left eye).

Fig. 2
At 16 months after the diagnosis of vernal keratoconjunctivitis, the conjunctival cyst on the limbus, which was not identified before, was detected (lined-arrow), It appears as a raised, circumscribed, pale, transparent cystic lesion and was located on the superior limbus. The size of the cysts in the left eye was larger than that of the right eye (A: Right eye, B: Left eye).

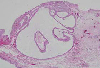
Fig. 3
The gross histopathologic finding disclosed the portion of conjunctiva showing a cystic wall lined by a single layer of low cuboidal epithelium with edema and congestion measuring 0.1-0.2 ml in volume. (H & E stain, ×100)
References
1. Trocme SD, Sra KK. Spectrum of ocular allergy. Curr Opin Allergy Clin Immunol. 2002. 2:423–427.
2. BenEzra D, Pe'er J, Brodsky M, Cohen E. Cyclosporin eyedrops for the treatment of severe vernal keratoconjunctivitis. Am J Ophthalmol. 1986. 101:278–282.
3. Grossniklaus HE, Green WR, Luckenbach M, Chan CC. Conjunctival lesions in adults. A clinical and histopathologic review. Cornea. 1987. 6:78–116.
4. Jakobiec FA, Bonanno PA, Sigelman J. Conjunctival adnexal cysts and dermoids. Arch Ophthalmol. 1978. 96:1404–1409.
5. Ho VT, Rao VM, Flanders AE. Postsurgical conjunctival epithelial cysts. AJNR Am J Neuroradiol. 1994. 15:1181–1183.
6. Bourcier T, Monin C, Baudrimont M, et al. Conjunctival inclusion cyst following pars plana vitrectomy. Arch Ophthalmol. 2003. 121:1067.
7. Finqer PT, McCormick SA, Lombardo J, et al. Epithelial inclusion cyst of the iris. Arch Ophthalmol. 1995. 113:777–780.
8. Srinivasan BD, Jakobiec FA, Iwamoto T, Devoe AG. Epibulbar mucogenic subconjunctival cysts. Arch Ophthalmol. 1978. 96:857–859.
9. Kiratli H, Bilgic S, Gokoz O, Sokmensuer C. Conjunctival epithelial inclusion cyst arising from a pterygium. Br J Ophthalmol. 1996. 80:769–770.
10. Suzuki K, Okisaka S, Nakagami T. The contribution of inflammatory cell infiltration to conjunctival inclusion cyst formation. Nippon Ganka Gakkai Zasshi. 2000. 104:170–173.
11. Jones BR. Trachoma and allied infections. Trans Ophthalmol Soc U K. 1961. 81:2115–2128.
12. Kim YH, Oh SY, Ha JK, Shin MC. Amniotic membrene transplantation in the management of shield ulcers of vernal keratoconjunctivitis. J Korean Ophthalmol Soc. 2004. 45:315–318.
13. Udell IJ, Gleich GJ, Allansmith MR, et al. Eosinophil granule major basic protein and Charcot-Leyden crystal protein in human tears. Am J Ophthalmol. 1981. 92:824–828.
14. Trocme SD, Kephart GM, Bourne WM, et al. Eosinophil granule major basic protein deposition in corneal ulcers associated with vernal keratoconjunctivitis. Am J Ophthalmol. 1993. 115:640–643.
15. Johnson DW, Bartley GB, Garrity JA, Robertson DM. Massive epithelium lined inclusion cysts after scleral buckling. Am J Ophthalmol. 1992. 113:439–442.
16. Soong HK, Okayawa RT, Iliff NT. Corneal astigmatism from conjunctival cyst. Am J Ophthalmol. 1982. 93:118–119.
17. de Bustros S, Michels RG. Treatment of acquired epithelial inclusion cyst of the conjunctiva using the YAG laser. Am J Ophthalmol. 1984. 98:807–808.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download