Abstract
Purpose
The purpose of this study was to identify differences in signal transduction gene expression between normal and diabetic keratocytes stimulated with interleukin-1α (IL-1α) and tumor necrosis factor-α (TNF-α).
Methods
Normal and diabetic keratocytes were primarily cultured and treated with 20 ng/ml IL-1α and TNF-α for 6 h. cDNA was hybridized to an oligonucleotide microarray. Genes identified by the microarray were further evaluated by real-time PCR.
Results
Diabetic keratocytes over-expressed components of the MAPK and Notch pathways, and under-expressed components of the insulin, calcium, and TGF-β pathways. Cytokine treated diabetic keratocytes differentially expressed components of the TGF-β and MAPK pathways. After IL-1α and TNF-α treatment, nine genes were under-expressed, falling in the insulin, TGF-β and Toll-like receptor pathways. Real-time PCR showed a significant decrease in the IL-6 and TGF-β 2 genes and a significant increase in the Ppm1a gene.
Conclusions
There were some differences in gene expression between normal and diabetic keratocytes related to signal transduction pathways, such as the insulin, MAPK, calcium, and TGF-β pathways. In addition, IL-1α and TNF-α stimulating the insulin, TGF-β, and Toll-like receptor signaling pathways may have different effects in diabetic keratocytes.
In diabetics, abnormalities are seen in the retina, lens, lid, iris, ciliary body, and nerves.1,2 Diabetic corneal complications, such as abnormalities in the basement membrane of the corneal epithelium, barrier function impairment in the corneal epithelium, decreased corneal perception, abnormal collagen depositions in Descemet′s membrane, morphological changes in corneal endothelial cells, and pump function deterioration are also common.3-6 Hemidesmosomes and anchoring fibrils passing through the stroma in the adhesion complex are reduced, resulting in weakened hemidesmosome adhesion. As a result, preservation of the corneal epithelial layer (corneal epithelialization) is impaired. Furthermore, abnormalities in the corneal epithelial basement membrane may impair normal barrier function or induce edema in the corneal stromal area. Thus, depending on the healing of wound and inflammation there may be substantial impediments to tissues recovery.3,4 In addition, as the corneal endothelial cell pump function is damaged by diabetes, water is transported to the anterior corneal stromal tissues; therefore, particular cautions are required to reduce corneal complications such as keratitis.5,6
Cells respond to external stimulation by transducing extracellular signals from cell membrane receptors to cytoplasmic micromolecules. All biological activities of cells (apoptosis, proliferation, migration, polarity, and so on) are determined by various signal transduction pathways. Therefore, studies pertinent to signal control are very important.7-10 In diabetes, morphological studies and reports on corneal cells are readily available; however, genetic and molecular studies on corneal stromal cells are relatively rare.
Furthermore, tissues react differently to cytokines in normal individuals, versus those with chronic diseases, such as diabetes, hypertension, or tumor lesions. These differences arise through differences in signal transduction pathway activation. We used microarrays and real-time polymerase chain reaction (real-time PCR) to measure the expression of genes or proteins involved in corneal stromal cell signal transduction. We compared normal cells in the spontaneously generated diabetes animal model (OLETF), with and without induction by pro-inflammatory cytokines.
Fifty-week-old genetically-affected type 2 diabetic (OLETF, Otsuka Long-Evans Tokushima Fattay, Japan) and normal rat eyes were purchased from Otsuka pharmaceutical company (Japan). The corneal epithelium was removed, the corneal stroma isolated, and washed several times in saline containing antibiotics. The corneal endothelial layer was removed, and the corneal stromal tissue was cut into small pieces, and cultured serially in culture medium. The culture medium was Dulbeco's Modified Eagle medium (DMEM, Gibco BRL, NY, USA) containing 10 % fetal bovine serum(FBS, Gibco BRL. NY, USA), 100 units/ml penicillin (Gibco BRL, NY, USA), and 100 mg/ml streptomycin (Gibco BRL. NY, USA). The culture medium was changed at 2-3 day intervals. When the cells became confluent, the culture medium was removed, the cells were washed once with Dulbeco's Phosphate-Buffered Saline (D-PBS, Gibco BRL, NY, USA) and dissociated with 0.25 % trypsin-0.02 % EDTA. For microarray experiments, cells between the 3rd and 4th generation of serial culture were collected and stored at -70℃.
The cytokines IL-1α (R&D Systems, MN, USA) and TNF((R&D Systems, MN, USA) were used to treat the corneal stromal cells of diabetic and normal rats treated at a concentration of 20 ng/ml for 6 hours.
Total cellular RNAs were extracted from the corneal stromal cells of diabetic and normal rats in primary culture using the RNeasy mini kit (QIAGEN inc, 74104, USA) for gene microarrays. First, denaturing solution was added to cell pellets on ice for 5 min, then phenol and chloroform were added to the samples, they were centrifuged, and the supernatant containing RNA was transferred to a new tube. The process was repeated, and subsequently, -20℃ isopropanol was added to precipitate the RNA, centrifuged, and the supernatant discarded. The pellet was washed with -20℃ 80 % ethanol and air-dried. The RNA was quantitated and confirmed by electrophoresis.
Sixty-mer oligonucleotides corresponding to each gene were synthesized, and subsequently placed on a slide using a robotic gene microarray. The robotic gene microarray places 0.25-1 nl DNA samples on a slide sample in spots averaging 100-150 nm. cDNA was synthesized from the isolated RNA samples using RT primers (Genisphere Inc, PA, USA) and SuperScript II reverse transcriptase (Invitrogen, NY, USA). The purified cDNA was hybridized to an Agilent Rat oligo 22K chip (Agilent tech, G4130A, USA). For hybridization, slides were placed in a dark hybridization chamber at 62℃ for 16 h. The slides were removed, washed 3 times, and dried using a centrifuge. A fluorescent dye was added to DNA capture reagents that bind RT primers, and a second hybridization was performed for 4 h. The slides were removed, washed, and dried using a centrifuge. Afterward, the fluorescence level was measured using an Axon laser fluorescence scanner (Axon ins, CA, USA). The microarray analyzer used in our study has 105 rows and 215 columns. A total of 22575 genes pertinent to the signal transduction were included.
The hybridized slides were scanned by an Axon GenePix Laser Fluorescence Scanner and analyzed by GenePix Pro 5.1 (Axon, CA, USA) and GeneSpring 7.0 (Silicongenetics, CA, USA). Genes showing more than a two-fold difference in expression level between normal and diabetic rats were classified as significant. Genes showing similar patterns were distinguished by a Pearson correlation. The images show a gene as red if it is up-regulated in the diabetic rat, and green if it is down-regulated.
Real-time polymerase chain reaction (real time-PCR) was performed with SYBR Green I immunofluorescence dye and the HotStarTag DNA polymerase QuantiTect SYBR Green PCR Kit (QIAGEN GmbH, Germany). To quantitate the control group, a standard curve of genes to be tested and GAPDH was prepared. The reactions were carried out in an ABI PRISM 7900 Sequence Detection System version 1.6 software (Perkin-Elmer Biosystems, Foster City, CA, USA). Relative quantitation measurements were performed applying the relative standard curve method according to the manufacturer's instructions.
The rats used in our experiments were 50-week-old Otsuka Long-Evans Tokushima Fatty (OLETF) diabetic rats. The blood glucose was an average of 115.0±0.8 mg/dL in the control group and 193.8±8.9 mg/dL in the OLETF group, with a significant difference. The body weights were 518.0±3.3 g in the control group, and 628.5±3.2 g in OLETF rats. Thus, classic diabetes was observed.
A total of 33 genes showed different expression in diabetic corneal stromal cells compared to normal cells. These genes are involved in the MAPK, calcium, TGF-β, Notch, phosphatidylinositol, Wnt, and Jak-STAT signal transduction pathways. In the insulin pathway, the cAMP-dependent regulatory type 1 α protein kinase and fatty acid synthase 2 were up-regulated, and 7 genes including the growth factor receptor bound protein 2, SHC transforming protein 1, and flotillin 1 were down-regulated. In the MAPK pathway, 7 genes including magnesium-dependent protein phosphatase 1A, platelet derived growth factor α, and V-jun (sarcoma virus 17 oncogene homolog) showed a difference in expression, all of which were up-regulated. In the calcium pathway, guanine nucleotide binding protein α 15 was up-regulated, while the adenosine A2B receptor, ATPase Ca++ transporting plasma membrane 1, and calcium channel voltage dependent L type genes were down-regulated. In the TGF-β pathway, BMP 4, decorin, inhibin β B, CREB binding protein, and thrombospondin 2 genes were down-regulated. In the Notch pathway, the hairy, enhancer of split 1, and Inicastrin genes were up-regulated. The genes involved in the Wnt signaling pathway were up-regulated. The inositol (myo)-1(or 4)-monophosphatase 1 and LIM domain kinase 2 genes in the phosphatidylinositol signal pathway and the prolactin gene in the Jak-STAT pathway were down-regulated (Table 1).
After cytokine treatment, there were differences between normal and diabetic cells in genes related to the TGF-β, MAPK, insulin, Phosphatidylinositol, Wnt, calcium, and Notch signal pathways. None of the Jak-STAT pathway genes, including prolactin, were different. Three genes were unresponsive to TNF-α and responded to IL-1α ; The Sp1 transcription factor (TGF-β pathway) and Peptidylprolyl isomerase D (calcium pathway) were down-regulated, and the Secreted frizzled related synthase 1 gene (Wnt pathway) was up-regulated. Several genes were unresponsive to IL-1α and responded to TNF-α ; Thrombospondin 4 (TGF-β), cell division cycle 2 homolog A and Angiotensin II type-I receptor gene (calcium), CDP diacylglycerol synthase 1 gene (phosphatidylinositol) were expressed at lower than normal levels, and the FBJ murine osteosarcoma viral oncogene homolog and Jun D proto-oncogene gene (MAPK) were up-regulated (Table 2).
IL-1α treatment induced expression of 14 genes belonging to the Jak-STAT, Toll-like receptor, calcium, MAPK, Wnt, and insulin pathways. Six genes were up-regulated, including Sphingosine kinase 1 (calcium), CD38 antigen gene, Neutrophic tyrosine kinase II (MAPK), Nuclear receptor subfamily 4 group A member 1 gene and Axin 2 (Wnt), and the Wnt inhibitory factor 1 gene. Eight genes were down-regulated, primarily in the Toll-like receptor, insulin, and Jak-STAT pathways (Table 3).
TNF-α treatment induced expression of genes in the calcium, MAPK, insulin, Toll-like receptor, Wnt, and Notch signal pathways. The MAPK, Wnt, and Notch pathways were up-regulated, while the calcium, insulin, and Toll-like receptor pathways were down-regulated. All genes were down-regulated (Table 4).
IL-1α and TNF-α stimulation both induced expression of nine genes in the insulin, TGF-β, Toll-like receptor, and adipocytokine pathways. In the insulin signal pathway, V-srk sarcoma virus CT 10 oncogene, suppressor of cytokine signaling 2, and liver glycogen phophorylase gene were expressed and down-regulated compared to control.
The follistatin (TGF-β), TGF-β 2, BMP 2, IL-6, and chemokine ligand 5 genes (Toll-like receptor), and Neuropeptide Y (adipocytokine) were down-regulated (Table 5).
Real-time PCR was performed on three genes that differed significantly between normal and diabetic rats, or were induced by cytokine treatment. The genes selected were IL-6, Ppm 1A, and TGF-β 2. The primer sets and annealing temperatures are shown in Table 6. These genes showed up- or down-regulation identical to the gene microarray results. For IL-6 and TGF-β 2, no significant difference between diabetic and normal cells was seen. After cytokine treatment, they showed significant down-regulation. The Ppm 1A gene showed a significant up-regulation in diabetes, as compared to the control and was up-regulated by cytokine treatment(Fig. 1).
In multicellular organisms, biological activities are controlled by cellular signals. Diverse signal transduction pathways regulate apoptosis, proliferation, migration, polarity, and other processes.10 In corneal tissues, the interaction between the stromal and epithelial layers plays an important role in proliferation, migration, differentiation, morphogenesis, and other functions. Furthermore, it has been shown to contribute to homeostasis in the ocular surface and wound healing.11,12
Abnormal signal transduction between the stroma and epithelial layers may result in impaired corneal tissue development or delayed wound healing. In diabetics, reduced corneal perception, an abnormal corneal epithelial basement complex, and impaired corneal epithelium barrier function develop. Furthermore, spot-like corneal epithelial defects, delayed corneal epithelium regeneration after trauma, and neurotrophic keratitis are common as well.3-6 Therefore, studies on genes pertinent to corneal signal transduction pathways are important for understanding the mechanism of corneal diseases induced by diabetes.
In our study, the expression of genes associated with signal transduction in normal corneal stroma cells was compared to expression in diabetic corneal stromal cells. The purpose of this study is to provide a basis of gene therapy by identifying gene alterations induced by diabetes. In addition, we compared genes related to signal transduction following cytokine stimulation. Responses to inflammatory reactions were examined using IL-1α and TNF-α
Generally, when the corneal epithelial layer is damaged, the tissue secretes cytokines. While corneal stromal cells undergo apoptosis, the cytokines induce chemotaxis, proliferation of activated stromal cells, and excessive secretion of extracellular matrix substances such as collagen, eventually resulting in corneal wound recovery. IL-1α and TNF-α are representative of the cytokines involved in this process.9,13,14 Because the cytokines are primarily secreted from damaged corneal epithelial cells,14 corneal stromal cells were exposed to IL-1α and TNF-α for approximately 6 hours. This time is sufficient to mimic the effect of cytokines released in response to corneal epithelial layer damage. Genes expressed at that time were classified as normal or diabetic and compared with each other.
The insulin, MAPK, calcium, and TGF-β pathways showed a large difference in expression between normal and diabetic corneal stromal cells. It has been shown that the insulin pathway improved the migration capacity of cells and thus accelerated wound healing instead of suppressing cell proliferation, following epithelial defect in corneal epithelial cells.15 In other words, the delayed wound healing that has been detected frequently in diabetes could be explained by abnormal insulin signaling. In our study, 5 of 6 insulin signal transduction genes were down-regulated.
The MAPK signal pathway has 3 subtypes, the c-jun N-terminal kinase (JNK) pathway, the extracellular signal-regulated kinase (ERK) pathway, and the p38 MAPK pathway. Upon activating the MAPK pathway, the tight junction in corneal epithelial cells is destroyed. Thus the pathway plays an important role in morphological control and corneal epithelial cell barrier function.16,17 All 7 genes involved in the MAPK signal pathway were up-regulated in diabetic corneal stromal cells.
Changes, such as cell migration and proliferation, are required for wound healing upon corneal epithelium damage requiring signal transduction among cells. Calcium-dependent signal transduction is required to convert extracellular stimulation to intercellular signal transduction. Calcium levels are increased in corneal epithelial cells in response to mechanical damage, transducing the signal to adjacent cells, thus facilitating wound healing.18,19 Therefore, our study suggests, that wound healing is delayed when corneal tissues are damaged in diabetics, because calcium signal transduction genes are down-regulated. It is thought that a deficiency of calcium-dependent signal transduction between adjacent cells may delay wound healing.
The TGF-β receptor is up-regulated after corneal wound, suggesting that the TGF-β signal pathway may play a role in repairing corneal epithelial cells.20 In addition, TGF-β controls corneal stromal cell and myofibroblast differentiation. Thus it has a role in scar formation in the wound healing process.21 Because the expression of pertinent TGF-β genes was decreased markedly, delayed wound healing in diabetes may be associated with the abnormal TGF-β signaling.
In the drosophila eye, Notch induced cell proliferation, but blocked cell differentiation in the primordial. The expression of Notch-related genes was up-regulated in diabetes.22 Although the cell proliferation occurs to heal corneal tissue wounds, the cell differentiation is not overly active, as to impair the normal eye surface.
The Jak-STAT pathway mediates the action of growth factors such as epidermal growth factor. Prolactin, a Jak-STAT gene, expression was decreased.23,24 This may explain why cell proliferation at the corneal surface layer is not active in diabetes.
Genes associated with the TGF-β MAPK, insulin, phosphatidylinositol, Wnt, calcium, and Notch signal pathways reacted to IL-1α and TNF-α treatment. Among genes reactive to IL-1α Sp1 transcription factor (TGF-β) and peptidylprolyl isomerase D (calcium) were down-regulated, and secreted frizzled related protein 1 (Wnt) was up-regulated. All were unresponsive to TNF-α. Secreted frizzled related protein 1 had a distinct expression level. It has been shown to play a role in controlling axonal growth in retinal ganglion cells and impeding the wound healing process. Thus it is thought to be associated with delayed wound healing in diabetes.25,26 Thrombospondin 4 (TGF-β), cell division cycle 2 homolog A and angiotensin II type-I receptor (calcium), and CDP diacylglycerol synthase 1 (phosphatidylinositol) were down-regulated, and FBJ murine osteosarcoma viral oncogene homolog and Jun D proto-oncogene gene (MAPK) were up-regulated in response to TNF-α and unresponsive to IL-1α. The angiotensin II type-I receptor gene showed a marked reduction that is primarily associated with delayed wound healing after conjunctival injury. It has been reported that the expression is decreased in diabetes, thus delaying wound repair.27,28
Genes newly expressed after IL-1α treatment were associated with the Toll-like receptor, calcium, MAPK, Wnt, insulin, and the Jak-STAT signal pathways. Sphingosine kinase 1, and CD38 antigen (calcium), Neutrophic tyrosine kinase II, and Nuclear receptor subfamily 4 group A member 1 (MAPK), and axin 2, and Wnt inhibitory factor 1 (Wnt) were up-regulated. Reduced expression was associated with the Toll-like receptor, insulin, Jak-STAT pathways. Toll-like receptor 2 and mitogen activated protein kinase 6 were down-regulated.
The Toll-like receptor signal pathway was especially involved following cytokine treatment. This pathway has been shown to be involved in the host defense mechanism. In other words, it is involved in both innate and acquired immune responses.29 According to our results, the expression of all genes associated with the Toll-like receptor was reduced in diabetes following inflammatory reaction. In diabetics the in vivo immune system reaction to cytokines is decreased; thus, in diabetes, the inflammatory response is weak. The host innate immune response is involved in infectious keratosis, and thus decreased noticeably in response to cytokines.30
In diabetes, genes associated with the calcium, MAPK, Notch, Toll-like receptor, insulin, and Wnt signal pathway were newly expressed after TNF-α treatment. The MAPK, Notch, and Wnt pathways were up-regulated, and the calcium, insulin, and Toll-like receptor pathways were down-regulated. Gene expression was decreased in all cases. Interferon β 1 and phospholamban showed marked down-regulation. Interferon β 1 has been reported to elevate anti-viral effects in the cornea. In diabetes with a concomitant inflammatory reaction, a reduced anti-viral effect can be detected due to immune system damage.31
Genes associated with the insulin, TGF-β, Toll-like receptor, and adipocytokine pathways were newly expressed in response to both IL-1α and TNF-α. The V-crk sarcoma virus CT 10 oncogene, suppressor of cytokine signaling 2, and liver glycogen phosphorylase (insulin), follistatin, TGF-β2, and BMP 2 (TGF-β, IL-6 and chemokine ligand 5 (Toll-like receptor), and Neuropeptide Y (adipocytokine) were down-regulated. Reduced Neuropeptide Y expression may delay angiogenesis and thus prolong wound healing.32
We further characterized several genes by real-time PCR, including Ppm 1A, which showed significant up-regulation after cytokine treatment. We also characterized TGF-β 2 and IL-6, which were down-regulated by cytokine treatment. The expression pattern was identical to the microarray analysis results.
By applying a microarray, we were able to observe differentially expressed genes pertinent to signal transduction pathways between normal and diabetic corneal stromal cells. By examining up- or down-regulated genes in diabetes, the signal transduction pathways involved in delayed wound healing and inflammatory response could be assessed. By looking at gene expression in response to interleukin or tumor necrosis factor, it is anticipated that genetic treatments for diabetic keratopathy and to prevent delayed corneal wound healing may be possible in the future.
Figures and Tables
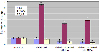 | Fig. 1Quantitative real-time PCR of three genes using GAPDH as an endogenous control. "*" indicates a significant difference between normal and diabetic rats stimulated with or without IL-1α or TNF-α IL-6 and TGF-β 2 showed no significant difference between untreated normal and diabetic rat, but the diabetic rat showed a significant down-regulation when cytokine treated. Ppm1a showed a significant up-regulation in all diabetic cases. |
Table 1
Up- and down-regulated genes in cultured diabetic rat keratocytes compared with normal rat keratocytes

Table 2
Up- and down-regulated genes involved in corneal keratocyte signal transduction expressed in diabetic rat following cytokine treatment

Table 3
Gene up- and down-regulated for newly expressed genes following interleukin-1α treatment in cultured diabetic rat keratocytes compared with normal rat keratocytes

Table 4
Gene up- and down-regulated for newly expressed genes following tumor necrosis factor-α treatment in cultured diabetic rat keratocytes compared with normal rat keratocytes

References
1. Aiello LP, Garaner TW, King GL, et al. Diabetic retinopathy. Diabetes Care. 1998. 21:143–156.
2. Herse PR. A review of manifestations of diabetes mellitus in the anterior eye and cornea. Am J Optom Physiol Opt. 1988. 65:224–230.
3. Inoue K, Kato S, Ohara C, et al. Ocular and systemic factors relevant to diabetic keratoepitheliopathy. Cornea. 2001. 20:798–801.
4. Foulks GN, Thoft RA, Perry HD, Tolentino FI. Factors related to corneal epithelial complications after closed vitrectomy in diabetes. Arch Ophthalmol. 1979. 97:1076–1078.
5. Dogru M, Katakami C, Inoue M. Tear function and ocular surface changes in noninsulin-dependent diabetes mellitus. Ophthalmology. 2001. 108:586–592.
6. Shultz RO, Van Hom DL, Peters MA, et al. Diabetic keratopathy. Trans Am Ophthalmol Soc. 1981. 79:180–199.
7. Msuda A, Suzuki Y, Honda G, et al. Large-scale identification and characterization of human genes that activate NF-κ B and Mapk signaling pathways. Oncogene. 2003. 22:3307–3318.
8. Cavllerano J. Ocular manifestation of diabetes mellitus. Optom Clin. 1992. 2:93–116.
9. Cubitt CL, Lausch RN, Oakes JE. Differences in interleukin 6 gene expression between cultured human corneal epithelial cells and keratocytes. Invest Ophthalmol Vis Sci. 1995. 36:330–336.
10. Singh SR, Chen X, Hou SX. JAK/STAT signaling regulates tissue outgrowth and male germline stem cell fate in Drosophilia. Cell Res. 2005. 15:1–5.
11. Khodadoust AA, Silverstein AM, Kenyon K, et al. Adhesion of regeneration corneal epithelium. Invest Ophthalmol Vis Sci. 1981. 21:317–321.
12. Wilson SE, Walker JW, Chwang EL, He YG. Hepatocyte growth factor (HGF), keratinocyate growth factor (KGF), their receptors, FGF receptor-2, and the cells of the cornea. Invest Ophthalmol Vis Sci. 1993. 34:2544–2561.
13. Wilson SE. Role of apoptosis in wound healing in the cornea. Cornea. 2000. 19:S7–S12.
14. Strissel KJ, Rinehart WB, Fini ME. A corneal epithelial inhibitor of stromal cell collagenase synthesis identified as TGF-β2. Invest Ophthalmol Vis Sci. 1995. 36:151–162.
15. Shanely LJ, McCaig CD, Forrester JV, Zhao M. Insulin, not leptin, promotes in vitro cell migration to heal monolayer wounds in human corneal epithelium. Invest Ophthalmol Vis Sci. 2004. 45:1088–1094.
16. Wang Y, Zhang J, Yi X, Yu FX. Activation of ERK1/2 MAP kinase pathway induces tight junction disruption in human corneal epithelial cells. Exp Eye Res. 2004. 78:125–136.
17. Luo L, Li D, Doshi A, et al. Experimental dry eye stimulates production of inflammatory cytokines and MMP-9 and activates MAPK signaling pathways on the ocular surface. Invest Ophthalmol Vis Sci. 2004. 45:4293–4301.
18. Klepeis VE, Cornell-Bell A, Trinkaus-Randall V. Growth factors but not gap junctions play a role in injury-induced Ca2+ waves in epithelial cells. J Cell Sci. 2001. 114:4185–4195.
19. Berridge MJ, Dupont G. Spatial and temporal signaling by calcium. Curr Opin Cell Biol. 1994. 6:267–274.
20. Zieske JD, Hutcheon AE, Guo X, et al. TGF-beta receptor types I and II are differentially expressed during corneal epithelial wound repair. Invest Ophthalmol Vis Sci. 2001. 42:1465–1471.
21. You L, Kruse FE. Differential effect of activin A and BMP-7 on myofibroblast differentiation and the role of the Smad signaling pathway. Invest Ophthalmol Vis Sci. 2002. 43:72–81.
22. Reynolds-Kenneally J, Moldzik M. Notch signaling controls proliferation through cell-autonomous and non-autonomous mechanisms in the Drosophila eye. Dev Biol. 2005. 285:38–48.
23. Tsai YC, Sun YH. Long-range effect of upd, a ligand for Jak-STAT pathway, on cell cycle in Drosophila eye development. Genesis. 2004. 39:141–153.
24. Zhong Z, Wen Z, Darnell JE. STAT3: a STAT family member activated by tyrosine and interleukin-6. Science. 1994. 264:95–98.
25. Rodringuez J, Esteve P, Weini C, et al. SFRP1 regulates the growth of retinal ganglion cell axons through the Fz2 receptor. Nat Neurosci. 2005. 8:1301–1309.
26. Li CH, Amar S. Role of Secreted Frizzled-related Protein 1 (SFRP1) in Wound Healing. J Dent Res. 2006. 85:374–378.
27. Kida T, Ikeda T, Nishimura M, et al. Renin-angiotensin system in proliferative diabetic retinopathy and its gene expression in cultured human muller cells. Jpn J Ophthalmol. 2003. 47:36–41.
28. Mizoue S, Iwai M, ide A, et al. Role of angiotensin II receptor subtypes in conjunctival wound healing. Curr Eye Res. 2006. 31:129–136.
29. Zhang J, Xu K, Ambati B, Yu FX. Toll-like receptor 5-mediated corneal epithelial inflammatory responses to pseudomonas aeruginosa flagellin. Invest Ophthalmol Vis Sci. 2003. 44:4247–4254.
30. Johnson AC, Heinzel FP, Diaconu E, et al. Activation of toll-like receptor (TLR)2, TLR4, and TLR9 in the mammalian cornea induces MyD88-dependent corneal inflammation. Invest Ophthalmol Vis Sci. 2005. 46:589–595.
31. Chen SH, Oakes JE, lausch RN. Synergistic anti-herpes effect of TNF-alpha and IFN-gamma in human corneal epithelial cells compared with that in corneal fibroblasts. Antiviral Res. 1994. 25:201–213.
32. Ekstrand AJ, Cao R, Bjorndahl M, et al. Deletion of neuropeptide Y (NPY) 2 receptor in mice results in blockage of NPY-induced angiogenesis and delayed wound healing. Proc Natl Acad Sci U S A. 2003. 100:6033–6038.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download