Abstract
Purpose
To establish the immortalized human corneal endothelial cell line (IHCEn) by transducing human papilloma virus (HPV) 16 E6/E7 oncogenes, and to identify their characteristics when cultivated on a lyophilized human amniotic membrane (LAM).
Methods
Primary human corneal endothelial cells (PHCEn) were infected using a retroviral vector with HPV 16 E6/E7, and transformed cells were clonally selected by G418. Growth properties and characteristics of IHCEn were compared with PHCEn by cell counting and RT-PCR of VDAC3, SLC4A4, CLCN3, FGF-1, Col IV, and Na+/K+ ATPase. IHCEn were cultured on LAM. Messenger RNA expressions of VDAC3, CLCN3, and Na+/K+ ATPase, and protein expressions of Na+/K+ ATPase and Col IV in IHCEn cultivated on LAM were investigated by RT-PCR, immunofluorescence, and immunohistochemical staining, respectively.
Results
Successful immortalization was confirmed by stable expression of HPV 16 E6/E7 mRNA by RT-PCR, and IHCEn exhibited typical corneal endothelial morphology. Doubling time of IHCEn was 30.15±10.96 hrs. Both IHCEn and PHCEn expressed VDAC3, CLCN3, SLC4A4, FGF-1, Col IV, and Na+/K+ ATPase. IHCEn cultivated on LAM showed stronger expression of VDAC3, CLCN4, and Na+/K+ ATPase mRNA than on plastic culture dish. Immunohistochemical staining and immunofluorescence revealed the positive expression of Na+/K+ ATPase and Col IV.
The corneal endothelium, a monolayer of differentiated cells located in the posterior portion of the cornea, is essential for maintaining corneal transparency by their dehydrating pumping action on the corneal stroma, and at least 1,500 corneal endotheial cells/mm2 are required for normal corneal function. The human corneal endothelium comprises a postnatal density of approx. 3,000 cells/mm.1,2 During life we experience a physiological reduction of cell density of about 0.5% per year,3 and endothelial cell loss can increase after intraocular surgery or in certain inherited diseases.4,5 Although once the density of endothelial cells, which have a limited regenerative capcity, reaches a critically low number, the integrity of the monolayer can be compromised, resulting in stromal edema, corneal opacity, and loss of visual acuity.6
Since a minimum cell density of 500 cells/mm2 is necessary to ensure a proper pump function, the only effective therapy to restore the corneal endothelium and thereby vision is corneal transplantation. Nevertheless, successful corneal transplantation also requires an optimum endothelial cell density, and donated corneas are very limited in Korea. To overcome these limitations, culturing human corneal endothelial cells on suitable biomaterials and transplanting the construct containing the intact endothelial monolayer to the posterior of the cornea was attempted.7-16 However, these experiments were limited by several problems: a lack of cell adherence after transplantation with insufficient substances, the use of non-human corneas, and insurance of a sufficient number of corneal endothelial cells.
Recently, the culture of corneal endothelial cells on biodegradable membranes17 and human corneal endothelium used for transplantation were genetically manipulated by transfection with the SV40 large T-antigen.18 One group also pursued an alternative strategy to increase the endothelial cell density by transplanting cultured corneal endothelial cells onto donor corneas, which were unsuitable for transplantation.19 Although these experiments reported a 80-90% graft success rate, the use of donated human cornea has a low efficiency and did not overcome the limitation of corneal transplantation. Therefore, two questions must be resolved for corneal endothelial cell transplantation.
First, the isolation of sufficient human corneal endothelial cells is essential for corneal endothelial cell transplantation. The most widely used method for immortalization of cells is the viral oncogene, SV40 large T-antigen, and immortalizations of corneal endothelial cells have been reported using human and murine cells.20,21 However, such cell lines had been shown to exhibit abnormal phenotypes, including a reduction of Na+/K+ pumping action and alterations of collagen expression. Recently, the establishment of more stable and physiologically relevant cell lines has been reported by transducing human papilloma virus (HPV) type 16 E6/E7 genes into various primary cells.2,3,5 E6 proteins are approximately 150 amino acids in length, and have the important function of binding the tumor suppressor p53, which results in its ubiquitin-mediated degradation.22 E7 proteins are approximately 100 amino acids in length, and associate with a member of the retinoblastoma tumor suppressor family to facilitate progression into S phase.23 Moreover, the E7 protein provides the synergistical transforming activity together with the E6 protein.24,25 Therefore, a more stable immortalization was expected by transducing with E6 and E7 in human corneal endothelial cells.
The second necessity for corneal endothelial cell transplantation is the development of a proper substrate sufficient not only for the corneal endothelial cells to adhere and grow, but also to transplant. As mentioned above, previously attempted substrates were abnormal corneal or biochemical materials. These substrates were not appropriate for the culture and transplantation of corneal endothelial cells, in addition to being very expensive and inefficient.
Human amniotic membrane, the innermost layer of human placenta, is harmless to humans and stimulates no immune reactions. The amniotic membrane also promotes wound healing and has a cell-protecting effect, which results in its clinical use in dermatology and ophthalmology for dressing wounds. Furthermore, the amniotic membrane has similar aspects to Descement's membrane. The major components of basement membrane are type IV, V, and VII collagen, and abundant extracellular matrix proteins including laminin and integrin. The use of amniotic membrane as a substrate and scaffold for the culture of corneal endothelial cells was expected to be successful.
Therefore, this experiment was conducted to establish immortalized human corneal endothelial cells (IHCEn) by stable introduction of HPV 16 E6/E7, and to investigate the biological characteristics of an established corneal endothelial cell line cultivated on a lyophilized human amniotic membrane.
All materials for cell culture including fetal bovine serum (FBS) and Opti-MEM were obtained from Gibco BRL (Grand Island, NY, USA). Other media supplements, such as epidermal growth factor (EGF), ascorbic acid, RPMI 1640 vitamin mixture, insect lipid, and chondroitin sulfate, were purchased from Sigma Chemical (St. Louis, MO, USA). Nerve growth factor (NGF) was obtained from R&D Systems (Minneapolis, MN, USA).
Human eyes were obtained in accordance with the tenets of the Declaration of Helsinki and proper informed consent. Human corneal endothelial cells were isolated by trypsin digestion of the posterior portions of excised cornea. After enzymatic digestion at 37℃ for 10 to 15 min, the endothelium was removed by gentle scraping, and seeded onto a tissue culture dish in Opti-MEM supplemented with 5 ng/ml of EGF, 20 ng/ml of NGF, 20 µg/ml of ascorbic acid, 0.005% insect lipid, 200 mg/l of CaCl2, 0.02% chondroitin sulfate, 1% RPMI 1640 vitamin mixture, and 8% FBS.
PA317 amphotropic packaging cell lines stably transfected with pLXSN 16 E6-E7 (HPV 16 E6/E7) were purchased from American Type Culture Collection (ATCC, Manassas, VA, USA). Cells were grown to 70-80% confluence, and supernatants were collected for 24 hr and stored in aliquots at -80℃. The primary endothelial cells were infected with 1 ml of virus stock in 3 ml of medium containing Polybrene (Sigma, MO, USA) at 4 µl/ml for 24 hr. The virus was then removed and the medium replaced by Opti-MEM supplemented with 8% FBS and other supplements as described above. Selection media with 200 µg/ml G418 (DUCHEFA, Amsterdam, Netherlands) was added after 72 hr. Cultures were maintained in selective media for two to three weeks. The G418-selected transformed cells were then grown and expanded further.
One of the established cell lines was seeded onto 100 mm-diameter culture dishes at a density of 5×104 cells/dish. Every two days for 14 days, cells were detached by 0.05% trypsin/0.5 mM ethylenediaminetetraacetic acid (EDTA), and counted in a hemocytometer in triplicate. For doubling time determination, the formula Tc=0.3T/log (A/A0) was used where Tc=doubling time, T=initial time, A=the number of cells at time T of proliferation, and A0=the number of cells at an initial time point (Table 1).
Human amniotic membrane from donated human placenta tested by hepatitis or HIV infection was pretreated with 0.025% trypsin-EDTA to remove amnion cells, followed by lyophilization and sterilization using EO gas. Lyophilized amniotic membranes (LAM) were installed on a Teflon ring, and 1×105 cells/support IHCEn were seeded onto the amnion side of a denuded LAM. Cells were maintained using opti-MEM for six days by changing the growth media every two days.
Corneal endothelial origin of the primary and transformed cells was confirmed by reverse transcription-polymerase chain reaction (RT-PCR). Primary cells and established cell lines were cultured as above, and total RNA was extracted with Trizol reagent (Gibco BRL, NY, USA). The first strand of cDNA was synthesized from two µg of the total RNA in a 20 µl reaction mixture containing Superscript II RNase, H reverse transcriptase, and oligo (dT)18 primer. Target genes (Table 2) were then amplified, PCR products were run on a preparative 2% agarose gel, and the bands were photographed.
An avidin biotin complex technique was selected for staining sectioned specimens (2-4 µm). Paraffin sections were deparaffinized in xylene, rehydrated through decreasing ethanol concentrations and quenched for endogenous peroxidase. Cryostat sections were placed on gelatinized slides, fixed in cold acetone and then rinsed in tris-buffered saline. Nonspecific background was eliminated by incubating tissue sections with non-immuno serum (Histostatin-plus Kits, Reagent A; Zymed Laboratories, CA, USA). Sections were then incubated with monoclonal mouse anti-human collagen IV (Santa Cruze Biotechnology Inc. CA, USA) and anti-human Na+/K+ ATPase antibody (Santa Cruze Biotechnology Inc. CA, USA) overnight at 4℃, followed by extensive washing in 0.05 M tris-buffered saline (pH 7.6) before the addition of a biotinylated secondary antibody (reagent B). Sections were washed again and incubated for one hour with peroxidase-conjugated streptavidin (reagent C). The presence of peroxidase was revealed by adding a substrate-chromogen (3-amino-9-ethycarbazole) solution (reagent D) for immunohistochemistry, and an FITC conjugated rabbit anti-mouse IgG for immunofluorescence. Sections were counterstained with haematoxylin. All sections were photographed and the entire tissue area was examined.
The morphological characteristics of isolated primary human corneal endothelial cells (PHCEn) and IHCEn were observed using an inverted microspcope. IHCEn were polygonal to slightly elongated in shape, similar to PHCEn, but were siginificantly different from dendritic corneal fibroblasts and small globular-shaped epithelial cells (Fig. 1). Stable expression of E6/E7 mRNA was observed in IHCEn, but not in PHCEn by RT-PCR analysis. Messenger RNA of the corneal epithelial cell marker, keratin 12, was not expressed in PHCEn and IHCEn (Fig. 2).
Proliferative characteristics of IHCEn were elucidated by cell counting every two days for 14 days. The cell growth curve of IHCEn showed typical S-curves: minimal growth for the initial seven days, geometrical growth until day 14, and decreased cell numbers after 14 days. The estimated cell doubling time of IHCEn was 30.15±10.96 hrs (Fig. 3). IHCEn showed a more extended life span (over passage 30), and more rapid proliferation than PHCEn (data not shown). Transformed traits, except for the transducing of HPV 16 E6/E7 oncogenes, were examined by RT-PCR of several channel proteins including voltage-dependent annion channel 3 (CDAC3), sodium bicarbonate cotransporter member 4 (SLC4A4), chloride channel protein 3 (CLCN3), fibroblast growth factor (FGF)-1, type IV collagen (Col IV), and Na+/K+ ATPase in PHCEn and IHCEn. mRNAs for all of the mentioned proteins were expressed in IHCEn, with similar expression patterns observed in PHCEn.
In order to elucidate the efficiency of LAM for the human corneal endothelial niche, IHCEn were cultured on LAM and harvested for characterization. Messenger RNA expressions of several channel proteins and Na+/K+ ATPase were observed in established IHCEn cultivated on LAM. Moreover, their expressions were stronger than in cells grown on a plastic culture dish (Fig. 5). Immunohitochemical staining of Col IV and immunofluorescence of Na+/K+ ATPase in IHCEn cultivated on LAM also showed positive expressions results similar to RT-PCR (Fig. 6).
Although the condition of the corneal endothelium is essential for corneal transparency, it is difficult to treat endothelial-damging corneal diseases, since the corneal cells have a very restricted regenerative capacity. In order to overcome these limitations, corneal endothelial cell transplantation had been attempted by several groups, but insurance of a sufficient number of endothelial cells and the development of appropriate endothelial substrates are still not guaranteed. Therefore, this experiment was conducted to establish immortalized human corneal endothelial cells (IHCEn) by stable introduction of HPV 16 E6/E7, and to investigate the biological characteristics of an established corneal endothelial cell line cultivated on lyophilized human amniotic membrane, an ideal coreneal endothelial substrate.
IHCEn stably transfected by PA317 amphotropic packaging cell lines with pLXSN 16 E6/E7 showed stable expression of E6/E7 mRNA. There was no corneal epithelial cell contamination observed by RT-PCR analysis of keratin 12, a corneal epithelial cell marker (Fig. 2). IHCEn showed morphological similarity with PHCEn, a longer life span (over passage 30), and more rapid proliferation than PHCEn (data not shown). Moreover, IHCEn showed a typical S-shaped growth curve by cell counting. The estimated cell doubling time of IHCEn, 30.15±10.96 hrs (Fig. 3), was faster than that of a previously established rabbit corneal endothelial cell line in our laboratory (51.30±7.3 hrs).26 Several immortalization techniques on human corneal endothelial cells by transfection of various oncogenes including SV40 large T-antigen or HPV 16 E6/E7 had been previously reported,27,28 but there were no reports elucidating the typical cell growth properties such as growth curve or doubling time.
Introduction of E6/E7 oncogenes facilitates the degradation of p53 and progression into the S phase synergistically.29-32 Moreover, their stability was also confirmed by observing which gene was non-carcinogenic when injected into nude mice.33 Therefore, we were able to successfully obtain abundant populations of highly proliferative and stably immortalized human corneal endothelial cells. Furthermore, it was also identified that established IHCEn maintained normal corneal endothelial functions similar to PHCEn by confirming VDAC3, CLCN3, CLC4A4, and Na+/K+ ATPase mRNA expressions (Fig. 4).
Corneal transparency is maintained by uniform structure, avascularity, and hydration of the stroma. The mechanism that maintains corneal hydration has an active component, HCO3-, which is transported from the stroma to the aqueous humor. A number of different hypotheses have been proposed to describe the molecular mechanisms that drive the net HCO3- flux. Common to these models is the recognition that the net HCO3- flux is coupled to and energized by a basolateral Na+/K+ ATPase in the corneal endothelium.34 The tendency to swell is due to the presence in the stromal matrix of non-diffusible, negatively charged molecules such as glycosaminoglycans. Ion transport across the endothelium is thought to involve the active transport of anions from the stroma towards the aqueous humor, followed by the mainly passive diffusion of cations. The osmotic effect of this transendothelial ion transport counters the excess osmotic potential of the stroma.34 At hydration levels outside the normal range, the cornea loses its transparency. Thus the positive expressions of several channel proteins including VDAC3 and Na+/K+ ATPase mRNA in IHCEn means that stably established IHCEn did not lose their normal endothelial functions, and can maintain them during ex vivo expansion.
Essential factors for normal endothelial functions such as channel proteins or Na+/K+ ATPase were positively expressed in cultivation not only on plastic culture dishes, but also on LAM. Furthermore, the expression patterns on LAM were stronger than that on plastic culture dishes. These results suggest that human amniotic membrane can act as the ideal endothelial niche for IHCEn and PHCEn.
The amniotic membrane has a specific advantage that makes it a good substrate for endothelial cell growth. It is a biomaterial different from biochemical substrates such as gelatin membrane or coated hydrolens, it increases cell adherence by abundant extracellular matrix proteins including integrin and lamin, and is easily transplanted since its physical properties are similar to Descement's membrane. Furthermore, our results demonstrate that amniotic membrane acts as a good substrate for the maintenance of corneal endothelial functions.
This study indicates that a sufficient immortalized cell line having characteristics similar to PHCEn is useful for in vitro studies of human corneal endothelial functions. Furthermore, it is expected that the culture of corneal endothelial cells on an amniotic membrane, an ideal corneal endothelial niche, is useful for the basic investigation of not only drug response or toxicological evaluation, but also for corneal endothelial cell transplantation or the development of reconstructed bioartificial cornea.
Figures and Tables
Fig. 1
The morphological characteristics of isolated primary human corneal endothelial cells (PHCEn) and immortalized human corneal
endothelial cells (IHCEn). IHCEn were polygonal to slightly elongate in shape, similar to PHCEn.

Fig. 2
Messenger RNA expressions of E6/E7 and keratin 12 in PHCEn and IHCEn. Successful immortalization was confirmed by stable expressions of E6/E7 oncogenes only in IHCEn. Both PHCEn and IHCEn were confirmed as endothelial in origin by negative RT-PCR results for keratin12.

Fig. 3
Cell growth curve of established IHCEn. 1×105 IHCEn cells/ml were counted every two days for 14 days. A geometrical increase in cell number was detected from day seven on. Doubling time of IHCEn was 30.15±10.96 hrs.

Fig. 4
Messenger RNA expressions of several channel proteins. VDAC3 (voltage-dependent annion channel 3), SLC4A4 (sodium bicarbonate cotransporter, member 4), CLCN3 (chloride channel protein 3), FGF (fibroblast growth factor)-1, Col IV (collagen type IV), and Na+/K+ ATPase in PHCEn and IHCEn were examined by RT-PCR. M denotes the 1Kb ladder. There were no differences in expression pattern between PHCEn and IHCEn.

Fig. 5
Messenger RNA expressions of channel proteins and Na+/K+ ATPase in established IHCEn cultivated on a lyophilized amniotic membrane (LAM) and plastic culture dish (PL), respectively. IHCEn cultivated on LAM showed slightly stronger expressions of all factors.
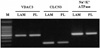
Fig. 6
Immunohistochemical staining of Col IV and Na+/K+ ATPase in IHCEn cultivated on LAM. (A) H&E staining, (B) immunohistochemical staining of Col IV, and (C) immunofluorescence of Na+/K+ ATPase. Established cell lines cultivated on LAM showed positive expressions of Col IV (arrow head) and Na+/K+ (green).

Notes
References
1. Maurice DM. The location of the fluid pump in the cornea. J Physiol. 1972. 221:43–54.
2. Murphy C, Alvarado J, Juster R, et al. Prenatal and postnatal cellularity of the human corneal endothelium. A quantitative histologic study. Invest Ophthalmol Vis Sci. 1984. 25:312–322.
3. Bourne WM, Nelson LR, Hodge DO. Central corneal endothelial cell changes over a ten-year period. Invest Ophthalmol Vis Sci. 1997. 38:779–782.
4. Bourne WM. Cellular changes in transplanted human corneas. Cornea. 2001. 20:560–569.
5. Bourne WM, Kaufman HE. Specular microscopy of human corneal endothelium in vivo. Am J Ophthalmol. 1976. 81:319–323.
6. Waring GO 3rd, Bourne WM, Edelhauser HF, et al. The corneal endothelium. Normal and pathologic structure and function. Ophthalmology. 1982. 89:531–590.
7. Alvarado JA, Gospodarowicz D, Greeburg G. Corneal endothelial replacement. In vitro formatin of an endothelial monolayer. Invest Ophthalmol Vis Sci. 1981. 21:300–316.
8. Gospodarowicz D, Greenburg G. The coating of bovine and rabbit corneas denuded of their endothelium with bovine corneal endothelial cells. Exp Eye Res. 1979. 28:249–265.
9. Gospodarowicz D, Greenburg G, Alvarado J. Transplantation of cultured bovine corneal endothelium on rabbit cornea: clinical implication for human studies. Proc Natl Acad Sci. 1979. 76:464.
10. Gospodarowicz D, Greenburg G, Alvarado J. Transplantation of cultured bovine corneal endothelium to species with nonregenerative endothelium. Arch Ophthalmol. 1979. 97:2163–2169.
11. Insler MS, Lopez JG. Extended incubation times improves corneal endothelial cell transplantation success. Invest Ophthalmol Vis Sci. 1991. 32:1828–1836.
12. Joyce NC, Meklir B, Neufeld AH. In vitro pharmacologic separation of corneal endothelial migration and spreading responses. Invest Ophthalmol Vis Sci. 1990. 31:1816–1826.
13. Jumblatt MM, Maurice DM, McCulley JP. Transplantation of tissue-cultured corneal endothelium. Invest Ophthalmol Vis Sci. 1978. 17:1135–1141.
14. Maurice DM, McCully JP, Perlmn MM. Development in use of cultured endothelium in corneal transplantation. Doc Ophthalmol Proc Ser. 1979. 20:151–153.
15. McCulley JP, Maurice DM, Schwartz BD. Corneal endothelial transplantation. Ophthalmology. 1980. 87:194–201.
16. Schwartz BD, McCully JP. Morphology of transplanted corneal endothelium derived from tissue culture. Invest Ophthalmol Vis Sci. 1981. 20:467–480.
17. Hadlock T, Singh S, Vacanti JP, et al. Ocular cell monolayers cultured on biodegradable substrates. Tissue Engng. 1999. 5:187–196.
18. Joo CK, Gree WR, Pepose JS, et al. Repopulation of denude murine Descement's membrane with life-extended murine corneal endothelial cells as a model for corneal transplantation. Graefe's Arch Clin Exp Ophthalmol. 2000. 238:174–180.
19. Engelmann K, Bednarz J, Valtink M. Prospects for endothelial transplantation. Exp Eye Res. 2004. 78:573–578.
20. Steinberg ML, Defendi V. Transformation and immortalization of human keratinocytes by SV40. J Invest Dermatol. 1983. 81:131S–136S.
21. Chang LS, Pan S, Pater MM, et al. Differential requirement for SV40 early genes in immortalization and transformation of primary rat and human embryonic cells. Virology. 1985. 146:246–261.
22. Wilson SE, Lloyd SA, He YG, et al. Extended life of human corneal endothelial cells transfected with the SV40 large T antigen. Invest Ophthalmol Vis Sci. 1993. 34:2112–2123.
23. Strauss M, Griffin BE. Cellular immortalization-an essential step or merely a risk factor in DNA virus-induced transformation? Cancer Cells. 1990. 2:360–365.
24. Wilson SE, Weng J, Blair S, et al. Expression of E6/E7 or SV40 large T antigen-coding oncogenes in human corneal endothelial cells indicates regulated high-proliferative capacity. Invest Ophthalmol Vis Sci. 1995. 36:32–40.
25. Fehrmann F, Laimins LA. Human papillomaviruses: targeting differentiating epithelial cells for malignant transformation. Oncogene. 2003. 22:5201–5207.
26. Shin JS, Jang IK, Kim CH, et al. Development and characterization of a rabbit corneal endothelial cell line. Jpn J Ophthalmol. 2004. 48:454–459.
27. Wilson SE, Weng J, Blair S, et al. Expression of E6/E7 or SV40 large T antigen-coding oncogenes in human corneal endothelial cells indicates regulated high-proliferative capacity. Invest Ophthalmol Vis Sci. 1995. 36:32–40.
28. He YG, Weng J, Li Q, et al. Fuch's corneal endothelial cells transduced with the human papilloma virus E6/E7 oncogenes. Exp Eye Res. 1997. 65:135–142.
29. Fehrmann F, Laimins LA. Human papillomaviruses: targeting differentiating epithelial cells for malignant transformation. Oncogene. 2003. 22:5201–5207.
30. Vousden KH. Interactions between papillomavirus proteins and tumor suppressor gene products. Adv Cancer Res. 1994. 64:1–24.
31. Bedell MA, Jones KH, Grossman SR, et al. Identification of human papillomavirus type 18 transforming genes in immortalized and primary cells. J Virol. 1989. 63:1247–1255.
32. Halbert CL, Demers GW, Galloway DA. The E7 gene of human papillomavirus type 16 is sufficient for immortalization of human epithelial cells. J Virol. 1991. 65:473–478.
33. Cole ST, Danos O. Nucleotide sequence and comparative analysis of the human papillomavirus type 18 genome. Phylogeny of papillomaviruses and repeated structure of the E6 and E7 gene products. J Mol Biol. 1987. 193:599–608.
34. Wigham CG, Turner HC, Swan J, et al. Modulation of corneal endothelial hydration control mechanisms by Rolipram. Pflugers Arch. 2000. 440:866–870.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download