Abstract
Purpose
We classified the Orbscan anterior elevation maps in normal eyes (under myopic, emmetropic and hyperopic conditions) and in those after myopic refractive surgery. We did this classification to demonstrate how Orbscan anterior elevation maps are useful in screening for the existence and extent of previous myopic refractive surgery. Such a classification can help clinicians interpret preoperative and postoperative topographies.
Methods
We measured for visual acuity and refractive power in 4800 eyes. After a slit-lamp examination, a corneal topography exam was performed with an Orbscan corneal topography system. The eyes were divided into two groups, with Group I representing those who had not had refractive surgery (4438 eyes). Group II included those who had undergone previous refractive surgery to correct myopia (362 eyes).
Results
In Group I, the central island type (43.0%) was the most common, followed by the temporal ridge (25.8%), the with-the-rule regular ridge (16.7%), the against-the-rule regular ridge (6.6%), the nasal ridge (4.0%), and the saddle type (2.1%). In Group II, the depressed lake type (69.9%) was most common, followed by the de-centered ablation type (21.3%). The trend line of the postoperative central anterior surface elevation (E) and the ablation power of refractive surgery were calculated. Ablation power of refractive surgery = 0.0047 E + 0.0083
Conclusions
This study demonstrates that it is possible to use Orbscan anterior elevation maps to screen for the extent of previous refractory surgery used in the correction of myopia. This study may also be useful in understanding the shapes of Orbscan anterior elevation maps before and after myopic refractive surgery as well as in determining the degree of ablated myopic refractive power and decentration.
If we do not know the refractive surgery history when calculating the intraocular lens (IOL) power for a cataract patient's eye, then an inaccurate IOL power makes for a low post-operative visual acuity. Repeated operations of refractive surgery for eyes cause corneal opacity and suppress corneal wound healing. In corneal transplantation, prior refractive surgery is also an exclusion criterion for corneal donation. Because an eye that has previously had refractive surgery may have the complications of decreased contrast sensitivity and night glare, the necessity of screening for previous refractive surgery is increasing for workers in specific fields, including pilots and delicate operators.1
Current excimer photoablations correct spherical myopic errors by removing a certain volume of the corneal tissue to flatten the central corneal surface.2 Although the wound of a laser in situ keratomileusis (LASIK) creates a flap, foreign materials under the LASIK flap and the anterior stromal opacity of the cornea can usually be detected by the slit-lamp. A thorough topography is needed to determine the extent of previous refractive surgery and also to conclude whether or not the eye has already been treated for myopia or hyperopia. In response to this, several studies have been conducted for the interpretation of corneal topography patterns in the normal eye1-4 or in eyes after refractive surgery.1,5-7 However, previous corneal topographies have limits, and even an experienced clinician can occasionally miss the diagnosis of a previous LASIK on an elevation or curvature map.
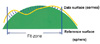
While corneal topography classification based on dioptric power maps2 and PAR-CTS (posterior apical radius-corneal topography systems)3 is well-known, classifications based on the Orbscan anterior elevation map have not been studied. By measuring 12,500 points on the anterior corneal surface, however, the Orbscan anterior elevation map provides more precise data than PAR-CTS in representing corneal morphology.1 (Fig. 1)
We measured for visual acuity and refractive power in 4800 eyes (mean age: 39.51±14.02 years) between January 2004 and March 2005. After a slit-lamp examination, a corneal topography was performed with the Orbscan IIz corneal topography system (Bausch & Lomb, USA; Fig. 2). Exclusion criteria included wearing contact lenses within two weeks before the examination, keratoconus, corneal disease including corneal opacity, severe pterygium, abnormal findings on the anterior segment, and eyes under 1.0 of corrected visual acuity.
The Orbscan is aligned with an axis of fixation, and the patient is instructed to fixate on a red flashing light during the examination. The anterior elevation is calculated with respect to the best-fit sphere (i.e. the sphere that best adjusts to the anterior surface of the cornea in the sense of the least mean square in an adjustment zone of 10.0 mm in diameter). All topographies were taken with the Orbscan anterior segment analysis system, which offers a best-fit sphere elevation and combines dual-slit scanning with Placido-disk technology.1
The eyes were divided into two groups. Group I represented those who had not had refractive surgery (4438 eyes) and Group II was made up of those who had had previous refractive surgery to correct myopia (362 eyes). Laser in situ keratomileusis (LASIK) was performed on all 362 eyes in Group II. Group I included myopic eyes (2836 eyes), emmetropic eyes (1292 eyes) and hyperopic eyes (310 eyes). The old charts of those in Group II were reviewed for refractive power, and Orbscan anterior elevation maps were collected before refractive surgery.
Using the Orbscan IIz anterior elevation maps (with an anterior best-fit sphere), Group I was divided into morphologic categories. The percentage of eyes falling into each category and the astigmatic diopters were calculated. Group II was also divided into morphologic categories.
We classified the morphology of Group I (eyes that had not been previously operated on: subgroups were myopic, emmetropic and hyperopic eyes) into six types, while Group II (eyes that had had previous refractive surgery) was divided into four types.
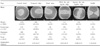
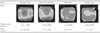
The classifications of Group I were "central island," "temporal ridge," "nasal ridge," "with-the-rule regular ridge," "against-the-rule regular ridge," "nasal ridge," and "saddle type" (Table 1). The classifications in Group II were "depressed lake," "decentered ablation," "flattened," and "normal type." The morphology of the "normal type" is similar to the morphology of Group I (Table 2).
The patients who had not had refractive surgery were divided into three subgroups according to refractive status (myopic, emmetropic and hyperopic eyes). A statistical analysis between subgroups was performed using the Mann-Whitney U-test (Statistica 5.0 for Windows, StatSoft, Inc.).
The percentage of eyes falling in each category and the ablation powers were then calculated, and statistical analyses were also performed. The mean value of the anterior surface elevation in a 0.5mm-radius central disk was calculated with Orbscan before and after refractive surgery in Group II. We also calculated the trend line of the central anterior surface elevation and the ablation power of refractive surgery. Pearson correlations were used to measure the linear relationship.
We classified the morphology of eyes with no previous history of surgery (myopic, emmetropic and hyperopic eyes) into six types, and designated the morphology of eyes that had undergone previous myopic refractive surgery into four types.
Orbscan anterior elevation maps of Group I were classified into six morphologic categories (Table 1). The central island type (43.0%) was the most common in all subgroups (myopic, emmetropic and hyperopic eyes), followed by the temporal ridge type (25.8%), the with-the-rule regular ridge type (16.7%), the against-the-rule regular ridge type (6.6%), the nasal ridge type (4.0%), and finally the saddle type (2.1%). There was no statistical significance between the subgroups in Group I (p=0.034). The central island type had the lowest mean astigmatism (0.43±0.37), followed by the temporal ridge type (0.59±0.27), the nasal ridge type (0.63±0.41), the with-the-rule regular ridge type (0.91±0.35), the against-the-rule regular ridge type (0.98±0.54), and the saddle type (1.42±0.81).
Orbscan anterior elevation maps of Group II were classified into four morphologic categories (Table 2). The depressed lake type (69.9%) was the most common and had the largest mean ablation power (-4.31±1.94 D), followed by the de-centered ablation type (21.3%), the flattening type (7.2%) and the normal type (1.7%). The mean ablation power also followed the same sequence as mentioned above, with the de-centered ablation type (-2.84±0.93 D), the flattening type (-1.73±0.49D) and the saddle type (-0.82±0.43 D) in descending order of frequency.
The mean value of the anterior surface elevation in a 0.5mm-radius central disk was calculated using the Orbscan both before and after refractive surgery in Group II. We also calculated the trend line of the postoperative central anterior surface elevation (E) and the ablation power of refractive surgery (Fig 3). The formula of the trend line is as follows:
Ablation power of refractive surgery = 0.0047 E + 0.0083
The Orbscan corneal topography system helps us to understand the corneal status after refractive surgery and also aids in diagnosing corneal disease by measuring its thickness and refractive power while producing an elevation map of the anterior and posterior surfaces of the cornea.
Before refractive surgery the normal cornea is prolate, meaning that the meridional curvature decreases from the center to the periphery. Immediately surrounding the central hill is an annular sea where the cornea dips below the reference surface. Anterior elevation maps of the Orbscan corneal topography system show a protruded central corneal shape and a flattened peripheral one. In the far periphery of Fig. 4, the prolate cornea again rises above the reference surface, producing a peripheral highland.
After myopic refractive surgery, the corneal surface is centrally flattened. An anterior elevation map of the Orbscan shows a flattened or depressed central corneal shape and a relatively elevated peripheral one. In Fig. 5, the relative elevation peak is not the highest point on the cornea. In fact, this apparent central concavity does not exist. After myopic refractive surgery, the postoperative central sea is not a concavity but a central flattening. The ring of seemingly higher terrain is not absolutely higher (more anterior) than the sea bottom near the map center.
This is the first research using anterior elevation maps of the Orbscan corneal topography system to determine and classify the morphology of normal eyes (myopic, emmetropic and hyperopic eyes) into six types and also to determine and classify the morphology of eyes that have undergone myopic LASIK surgery into four different types. The percentage of eyes falling into each category and the ablation power were calculated from these figures.
In Group I, the central island type was the most common, followed by the temporal ridge, the with-the-rule regular ridge, the against-the-rule regular ridge, the nasal ridge, and the saddle type. In Group II, the depressed lake type was the most common, followed by the de-centered ablation. If the ablation power was more than -2.50D, it represented a depressed lake type. However, if the ablation power was lower than -2.25D, it became a flattened corneal center type represented by a green color in the center. If the ablation power was lower than -1.25D, a normal corneal morphology appeared.
The mean pre-operative E value of Group II was 0.0072±0.0029, and no eyes had a pre-operative E value below zero. The mean post-operative E value of Group II was -0.0014±0.0085. There were six eyes that had a post-operative E value above zero. Their mean post-operative period was 14.3±6.4 months, and the mean ablation power was -0.82±0.43 D. After myopic refractive surgery, the generation of myopic regression was known. In this study, we assumed a positive E value and an insufficiently small negative E value (in the flattening type) that caused either post-operative regression or an insufficiently small ablation power.
A limitation of our study was a lack of subjects with an ablation power lower than -2.00 D. It would have been useful to study any changes in the Orbscan anterior elevation map with more subjects of low myopic refractive surgical ablation power.
There have been many studies that have dealt with screening for previous refractive surgery by using a slit-lamp,8 pachymetry9-10 or optical coherence tomography.11-12 In eyes that have had previous refractive surgery, pachymetry gives us necessary information about decreased corneal thickness, and ultrasonography warns us about unusual eye axial lengths that are too short or too long. However, we cannot jump to any conclusions about refractive surgery based solely on this information because even a normal eye with no previous surgeries can have variations. Although the wounds of a laser in situ keratomileusis (LASIK) form a flap, foreign materials under the LASIK flap and the anterior stromal opacity of the cornea can usually be detected by the slit-lamp. There are also many false negatives8 produced this way. Screening for previous refractive surgery is possible with optical coherence tomography, but it is particularly useful in the case of LASIK.11-12 In the case of PRK, however, this method produces many false negatives. Topography is needed to determine the degree of refractive surgery and whether the eye has previously been treated for myopia or hyperopia. Moreover, pachymetry and ultrasonography are difficult to use screen for previous refractive surgeries because of normal variations in the human eye.
As we now know, being unaware of a previous refractive surgical intervention can interfere with intraocular lens (IOL) power calculations and can also result in severe postoperative ametropia. By focusing only on the color of the central area of the Orbscan anterior elevation map, automated screening can be conducted by paramedical staff for previous refractive surgery prior to cataract surgery and for the selection of pilot applicants. Moreover, if we calculate the 0.5 mm-radius central anterior surface elevation (E) with Orbscan, we can determine the degree of ablation power used in previous myopic refractive surgery.
This study demonstrates that it is possible to use Orbscan anterior elevation maps to screen for the degree of power in previous refractory surgery that has been performed to correct myopia. This study may be useful in understanding the shapes of Orbscan anterior elevation maps before and after myopic refractive surgery as well as in determining the degree of ablated myopic refractive power and the degree of de-centration.
Figures and Tables
Fig. 3
Trend line about the 0.5mm radius central anterior surface elevation (E) and ablation power of refractive surgery.

References
1. Destrempes F, Brunette I, Meunier J, et al. Topography-based screening for previous laser in situ keratomileusis to correct myopia. J Cataract Refract Surg. 2002. 28:1644–1650.
2. Bogan SJ, Waring GO 3rd, Ibrahim O, et al. Classification of normal corneal topography based on computer-assisted videokeratography. Arch Ophthalmol. 1990. 108:945–949.
3. Naufal SC, Hess JS, Friedlander MH, Granet NS. Rasterstereography-based classification of normal corneas. J Cataract Refract Surg. 1997. 23:222–230.
4. Dingeldein SA, Klyce SD, Wilson SE. Quantitative descriptors of corneal shape derived from computer-assisted analysis of photokeratographs. Refract Corneal Surg. 1989. 5:372–378.
5. Hersh PS. A standardized classification of corneal topography after laser refractive surgery. J Refract Surg. 1997. 13:571–578.
6. Abbas UL, Hersh PS. Early corneal topography patterns after excimer laser photorefractive keratectomy for myopia. J Refract Surg. 1999. 15:124–131.
7. Hersh PS, Scher KS, Irani R. Corneal topography of photo-refractive keratectomy versus laser in situ keratomileusis. Summit PRK-LASIK Study Group. Ophthalmology. 1998. 105:612–619.
8. Mootha VV, Dawson D, Kumar A, et al. Slitlamp, specular, and light microscopic findings of human donor corneas after laser-assisted in situ keratomileusis. Arch Ophthalmol. 2004. 122:686–692.
9. Ousley PJ, Terry MA. Objective screening methods for prior refractive surgery in donor tissue. Cornea. 2002. 21:181–188.
10. Hjortdal JO, Moller-Pedersen T, Ivarsen A, Ehlers N. Corneal power, thickness, and stiffness: results of a prospective randomized controlled trial of PRK and LASIK for myopia. J Cataract Refract Surg. 2005. 31:21–29.
11. Priglinger SG, Neubauer AS, May CA, et al. Optical coherence tomography for the detection of laser in situ keratomileusis in donor corneas. Cornea. 2003. 22:46–50.
12. Wolf AH, Neubauer AS, Priglinger SG, et al. Detection of laser in situ keratomileusis in a postmortem eye using optical coherence tomography. J Cataract Refract Surg. 2004. 30:491–495.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download