Abstract
Purpose
To report a case of uveal effusion syndrome associated with hypotony and a case of uveal effusion syndrome in nanophthalmos.
Methods
The first case was a 25-year-old man who presented with decreased visual acuity in the left eye and hypotony. Fundus examination revealed choroidal effusion and retinal detachment with a thickened eyeball. Partial thickness sclerotomy and sclerectomy were performed. The second case was a 13-year-old boy who had uveal effusion syndrome with a nanophthalmic eye.
Results
In the patient with hypotony, intraocular pressure was well maintained following partial thickness sclerotomy and sclerectomy, and choroidal effusion and retinal detachment were reduced. The visual acuity of the nanophthalmic patient was well maintained during a 3-year follow-up period without treatment.
Uveal effusion was first described by Schepens and Brockhurst in 1963.1 In 1975 Brockhurst2,3 reported this disorder in association with nanophthalmos and scleral abnormality, and proposed that subretinal fluid accumulation is caused by congestion of the choroidal venous system due to compression of the vortex veins by thick sclera. Brockhurst subsequently described a new surgical procedure involving decompression of the vortex vein. In this paper we report two cases of uveal effusion. The first patient presented with severe hypotony and decreased visual acuity. He was diagnosed with uveal effusion and received subscleral sclerectomy and sclerotomy. The second patient had uveal effusion and nanophthalmos. He demonstrated decreased visual acuity initially, but did not experience any further change during a follow up period of three years.
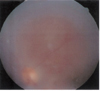
A 25-year-old male presented with blurred vision in his left eye that had persisted for 5 days. He also complained of eyeball pain. He had no relevant general medical or ocular history, and took no medication. His best corrected visual acuity (BCVA) in right eye was 1.0 and that of the left eye was finger count 50 cm. Intraocular pressure (IOP) was 12 mmHg in the right eye and 2mmHg in the left eye. Slit lamp examination showed cells 3+ in the left anterior chamber, posterior synechiae and posterior capsular opacity in the left eye. Fundus examination (Fig. 1) demonstrated shallow retinal detachment and choroidal folding in the left eye. No retinal breaks were found. Ultrasound examination (Fig. 2) revealed thickened sclera and choroidal detachment in the left eye. Fluorescein angiogram and indocyanine green (ICG) showed diffuse hyperfluorescence in the choroid that appeared in the early phase (10 seconds) and had almost disappeared in the late phase.
Administration of high-dose oral steroid and NSAID was not effective, therefore, high-dose intravenous steroid was administered. Subsequent IOP was 1 mmHg in the left eye and slit lamp examination showed cells 3+ in the left anterior chamber. No improvement was noted in the fundus examination. To correct the IOP, 0.7 cc 100% SF6 gas was injected into the vitreous; however the postoperative IOP remained 1 mmHg. Surgical treatment was therefore performed on the left eye under general anesthesia.
Subscleral sclerectomy was performed at two sites in the temporoinferior and nasoinferior quadrants. A half thickness scleral flap measuring 4×4 mm was made 8 mm from the limbus. Under the scleral flap, the remaining thickness of the sclera was excised in pieces measuring 2×2 mm, thereby exposing the choroid. The scleral flap was loosely sutured and C3F8 gas was injected into the vitreous. At postoperative 1 week, IOP in the left eye was 46 mmHg, and Diamox®, Cosopt®, Alphagan® and Xalatan® were administered. One week later the IOP in the left eye was 17 mmHg. At postoperative 2 months, fundus examination (Fig. 3) revealed an attached retina and near complete resolution of the choroidal detachment. Five months after surgery, IOP was 11 mmHg and BCVA was 0.1 in the left eye. No surgery-related side effects were noted and cataract surgery was considered to improve visual acuity.
A 13-year-old boy was referred to our hospital due to decreased visual acuity in both eyes over 2 years. He had no relevant general medical or ocular history, and took no medication. One year previously, brain MRI had revealed no abnormalities and an electoretinogram (ERG) showed decreased electrical signals. At presentation, BCVA was 0.5 for both eyes and both spherical equivalents were +7.00D (diopter). Slip lamp examination showed no specific features, while fundus examination revealed tortuous dilated retinal vessels, serous retinal detachment and subretinal fluid at periphery, and choroidal folding and epiretinal membrane in both eyes (Fig. 4). Axial lengths were 18.96 mm in the right eye and 18.99 mm in the left eye in A-scan. A B-scan ultrasonogram showed thickened choroid, retinal detachment and choroidal detachment (Fig. 5). During a 3-year follow up period, fundus examination showed no interval change and BCVA was 0.7 in the right eye and 0.6 in the left eye. He is currently under observation without any medication.
Schepens and Brockhurst1 first described uveal effusion, showing non-rhegmatogenous retinal detachment and subretinal fluid shift in 17 cases. Brockhurst2,3 later reported that this disorder is related to nanophthalmos and scleral abnormality, and proposed that subretinal fluid accumulation is caused by congestion of the choroidal venous system due to compression of the vortex veins by thick sclera. In 1983 Gass4 reported thick sclera and uveal effusion in non-nanophthalmic patients. He proposed a new hypothesis in which congenital choroidal abnormalities, such as thickened choroid and abnormal vortex veins, cause uveal effusion, and he introduced a surgical procedure involving scleral resection without decompression of the vortex vein.
Trelstad et al.5 found that in uveal effusion syndrome, the sclera showed histochemical abnormalities: The bundles of collagen fibers in the sclera had a markedly irregular arrangement and varied in width, and abnormal deposition of glycosaminoglycan among the collagen bundles was noted.6-8 Uveal effusion syndrome may be divided into three groups according to its pathogenesis: 1) Uveal effusion caused by intraocular inflammation due to ocular trauma, intraocular surgery and scleritis; 2) Uveal effusion caused by hypotony and thickened sclera; 3) Idiopathic causes. Uyama et al.7 reported 17 cases of uveal effusion syndrome and divided them into 3 groups. Type 1 was a nanophthalmic eye with axial length less than 19 mm, a high grade of hypermetropia in refraction, and a rigid, thick sclera. Type 2 was a non-nanophthalmic eye with thick sclera but no high degree of hypermetropia. Type 3 was a non-nanophthalmic eye without scleral thickening and a normal eyeball size Type 3 thus corresponded to idiopathic uveal effusion. They reported that types 1 and 2 showed similar clinical features and surgery was effective for both, while surgical treatment was not effective in type 3. They concluded that scleral abnormality is the main cause of primary uveal effusion syndrome in types 1 and 2. It is known that idiopathic uveal effusion has a poorer prognosis than other types, and that surgery or systemic steroid therapy is not effective.
In 1986, Oum9 reported subscleral sclerectomy in a case of uveal effusion syndrome in Korea. In 1996 Song et al.10 reported a case of nanophthalmos with choroidal effusion in which anterior sclerotomy for the drainage of the uveal effusion was effective.
We report two cases of uveal effusion. The first patient, who presented with severe hypotony and decreased visual acuity, was diagnosed with uveal effusion and received subscleral sclerectomy and subscleral sclerotomy. The second case was a patient with uveal effusion and nanophthalmos who initially showed decreased visual acuity, but did not experience any change during the follow up period of three years with no treatment.
In conclusion, in the management of uveal effusion, appropriate treatment modalities should be considered on an individual basis depending on the patient's ophthalmic condition.
Figures and Tables
 | Fig. 1Preoperative color photograph of case 1 showing shallow retinal detachments and choroidal folding in the left eye. |
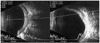 | Fig. 2B-scan ultrasound in case 1 showed thickened eyeball and choroidal detachment in the left eye. |
References
1. Schepens CL, Brockhurst RJ. Uveal effusion. I. Clinical picture. Arch Ophthalmol. 1963. 70:189–201.
2. Brockhurst RJ. Nanophthalmos with uveal effusion: a new clinical entity. Arch Ophthalmol. 1975. 93:1989–1999.
3. Brockhurst RJ. Vortex vein decompression for nanophthalmic uveal effusion. Arch Ophthalmol. 1980. 98:1987–1990.
4. Gass JDM. Uveal effusion syndrome: a new hypothesis concerning pathogenesis and technique of surgical treatment. Trans Am Ophthalmol Soc. 1983. 81:246–260.
5. Trelstad RL, Silbermann NN, Brockhurst RJ. Nanophthalmic sclera: ultrastructural, histochemical, and biochemical observations. Arch Ophthalmol. 1982. 100:1935–1938.
6. Stewart DH III, Streeten BW, Brockhurst RJ, et al. Abnormal scleral collagen in nanophthalmos: an ultrastructural study. Arch Ophthalmol. 1991. 109:1017–1025.
7. Uyama M, Takahashi K, Kozaki J, et al. Uveal effusion syndrome: clinical features, surgical treatment, histologic examination of the sclera, and pathophysiology. Ophthalmology. 2000. 107:441–449.
8. Young RD. The ultrastructural organization of proteoglycans and collagen in human and rabbit scleral matrix. J Cell Sci. 1985. 74:95–104.
9. Oum BS. Uveal effusion. J Korean Ophthalmol Soc. 1986. 27:1013–1017.
10. Song SW, Kwak NH, Huh W. A case of nanophthalmos. J Korean Ophthalmol Soc. 1996. 37:687–692.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download